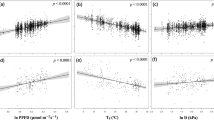
Abstract
Leaf thermoregulation has been documented in a handful of studies, but the generality and origins of this pattern are unclear. We suggest that leaf thermoregulation is widespread in both space and time, and originates from the optimization of leaf traits to maximize leaf carbon gain across and within variable environments. Here we use global data for leaf temperatures, traits and photosynthesis to evaluate predictions from a novel theory of thermoregulation that synthesizes energy budget and carbon economics theories. Our results reveal that variation in leaf temperatures and physiological performance are tightly linked to leaf traits and carbon economics. The theory, parameterized with global averaged leaf traits and microclimate, predicts a moderate level of leaf thermoregulation across a broad air temperature gradient. These predictions are supported by independent data for diverse taxa spanning a global air temperature range of ∼60 °C. Moreover, our theory predicts that net carbon assimilation can be maximized by means of a trade-off between leaf thermal stability and photosynthetic stability. This prediction is supported by globally distributed data for leaf thermal and photosynthetic traits. Our results demonstrate that the temperatures of plant tissues, and not just air, are vital to developing more accurate Earth system models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Michaletz, S. T. et al. Plant thermoregulation: energetics, trait-environment interactions, and carbon economics. Trends Ecol. Evol. 30, 714–724 (2015).
Mahan, J. R. & Upchurch, D. R. Maintenance of constant leaf temperature by plants—I. Hypothesis-limited homeothermy. Environ. Exp. Bot. 28, 351–357 (1988).
Michaletz, S. T., Cheng, D., Kerkhoff, A. J. & Enquist, B. J. Convergence of terrestrial plant production across global climate gradients. Nature 512, 39–43 (2014).
Cuny, H. E. et al. Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nat. Plants 1, 15160 (2015).
Yvon-Durocher, G. et al. Methane fluxes show consistent temperature dependence across microbial to ecosystem scales. Nature 507, 488–491 (2014).
Williams, P. A. et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Change 3, 292–297 (2013).
Huxman, T. E., Turnipseed, A. A., Sparks, J. P., Harley, P. C. & Monson, R. K. Temperature as a control over ecosystem CO2 fluxes in a high-elevation, subalpine forest. Oecologia 134, 537–546 (2003).
Berry, J. & Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Ann. Rev. Plant Physiol. 31, 491–543 (1980).
Slatyer, R. & Morrow, P. Altitudinal variation in the photosynthetic characteristics of snow gum, Eucalyptus pauciflora Sieb. Ex Spreng. I. Seasonal changes under field conditions in the Snowy Mountains area of south-eastern Australia. Aust. J. Bot. 25, 1–20 (1977).
Korner, C. & Diemer, M. In situ photosynthetic responses to light, temperature and carbon dioxide in herbaceous plants from low and high altitude. Funct. Ecol. 1, 179–194 (1987).
Yamori, W., Hikosaka, K. & Way, D. A. Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynth. Res. 119, 101–117 (2014).
Gates, D., Hiesey, W., Milner, H. & Nobs, M. Temperatures of Mimulus leaves in natural environments and in a controlled chamber. Carnegie Inst. Wash. Yearb. 63, 418–430 (1964).
Linacre, E. T. A note on a feature of leaf and air temperatures. Agric. Meteorol. 1, 66–72 (1964).
Linacre, E. T. Further notes on a feature of leaf and air temperatures. Arch. Meteorol. Geophys. Bioklimatol. B 15, 422–436 (1967).
Upchurch, D. R. & Mahan, J. R. Maintenance of constant leaf temperature by plants—II. Experimental observations in cotton. Environ. Exp. Bot. 28, 359–366 (1988).
Helliker, B. R. & Richter, S. L. Subtropical to boreal convergence of tree-leaf temperatures. Nature 454, 511–514 (2008).
Potter, K., Davidowitz, G. & Woods, H. A. Insect eggs protected from high temperatures by limited homeothermy of plant leaves. J. Exp. Biol. 212, 3448–3454 (2009).
Smith, W. K. & Carter, G. A. Shoot structural effects on needle temperatures and photosynthesis in conifers. Am. J. Bot. 75, 496–500 (1988).
Huey, R. B. & Slatkin, M. Cost and benefits of lizard thermoregulation. Q. Rev. Biol. 51, 363–384 (1976).
Rowland, L. et al. Modelling climate change responses in tropical forests: similar productivity estimates across five models, but different mechanisms and responses. Geosci. Model Dev. 8, 1097–1110 (2015).
Reich, P. B., Walters, M. B. & Ellsworth, D. S. From tropics to tundra: global convergence in plant functioning. Proc. Natl Acad. Sci. 94, 13730–13734 (1997).
Wright, I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827 (2004).
Falster, D. S. et al. Lifetime return on investment increases with leaf lifespan among 10 Australian woodland species. New Phytol. 193, 409–419 (2012).
Blonder, B., Violle, C., Bentley, L. P. & Enquist, B. J. Venation networks and the origin of the leaf economics spectrum. Ecol. Lett. 14, 91–100 (2011).
Ball, M., Cowan, I. & Farquhar, G. Maintenance of leaf temperature and the optimisation of carbon gain in relation to water loss in a tropical mangrove forest. Funct. Plant Biol. 15, 263–276 (1988).
Slot, M., Garcia, M. N. & Winter, K. Temperature response of CO2 exchange in three tropical tree species. Funct. Plant Biol. 43, 468–478 (2016).
Slot, M. & Winter, K. in Tropical Tree Physiology Adaptations and Responses in a Changing Environment (eds Goldstein, G. & Santiago, S. L. ) 385–412 (Springer International, 2016).
The Botanical Information and Ecology Network (BIEN, 2016); http://bien.nceas.ucsb.edu/bien/
New, M., Lister, D., Hulme, M. & Makin, I. A high-resolution data set of surface climate over global land areas. Climate Res. 21, 1–25 (2002).
Kearney, M. R., Isaac, A. P. & Porter, W. P. microclim: Global estimates of hourly microclimate based on long-term monthly climate averages. Sci. Data 1, 140006 (2014).
Paw U, K. T. A theoretical basis for the leaf equivalence point temperature. Agricult. Meteorol. 30, 247–256 (1984).
Kikuzawa, K. & Lechowicz, M. J. Toward synthesis of relationships among leaf longevity, instantaneous photosynthetic rate, lifetime leaf carbon gain, and the gross primary production of forests. Am. Nat. 168, 373–383 (2006).
Ehleringer, J. R. Changes in leaf characteristics of species along elevational gradients in the Wasatch Front, Utah. Am. J. Bot. 75, 680–689 (1988).
Moles, A. T. et al. Which is a better predictor of plant traits: temperature or precipitation? J. Veg. Sci. 25, 1167–1180 (2014).
Leigh, A. et al. Do thick leaves avoid thermal damage in critically low wind speeds? New Phytol. 194, 477–487 (2012).
Vogel, S. Leaves in the lowest and highest winds: temperature, force and shape. New Phytol. 183, 13–26 (2009).
Song, X., Barbour, M. M., Saurer, M. & Helliker, B. R. Examining the large-scale convergence of photosynthesis-weighted tree leaf temperatures through stable oxygen isotope analysis of multiple data sets. New Phytol. 192, 912–924 (2011).
Barbour, M. M., Andrews, T. J. & Farquhar, G. D. Correlations between oxygen isotope ratios of wood constituents of Quercus and Pinus samples from around the world. Funct. Plant Biol. 28, 335–348 (2001).
Saurer, M., Schweingruber, F., Vaganov, E. A., Shiyatov, S. G. & Siegwolf, R. Spatial and temporal oxygen isotope trends at the northern tree-line in Eurasia. Geophys. Res. Lett. 29, 7-1–7-4 (2002).
Evans, M. N. & Schrag, D. P. A stable isotope-based approach to tropical dendroclimatology. Geochim. Cosmochim. Acta 68, 3295–3305 (2004).
Poussart, P. F., Evans, M. N. & Schrag, D. P. Resolving seasonality in tropical trees: multi-decade, high-resolution oxygen and carbon isotope records from Indonesia and Thailand. Earth Planet. Sci. Lett. 218, 301–316 (2004).
Poussart, P. F. & Schrag, D. P. Seasonally resolved stable isotope chronologies from northern Thailand deciduous trees. Earth Planet. Sci. Lett. 235, 752–765 (2005).
Flanagan, L. B. & Farquhar, G. D. Variation in the carbon and oxygen isotope composition of plant biomass and its relationship to water-use efficiency at the leaf- and ecosystem-scales in a northern Great Plains grassland. Plant Cell Environ. 37, 425–438 (2014).
Barbour, M. M. & Farquhar, G. D. Relative humidity- and ABA-induced variation in carbon and oxygen isotope ratios of cotton leaves. Plant Cell Environ. 23, 473–485 (2000).
Boyle, B. et al. The taxonomic name resolution service: an online tool for automated standardization of plant names. BMC Bioinform. 14, 1–15 (2013).
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005).
Paw U, K. T. Mathematical analysis of the operative temperature and energy budget. J. Therm. Biol. 12, 227–233 (1987).
Tracy, C. R. et al. Errors resulting from linear approximations in energy balance equations. J. Therm. Biol. 9, 261–264 (1984).
Paw U, K. T. & Gao, W. Applications of solutions to non-linear energy budget equations. Agric. Forest Meteorol. 43, 121–145 (1988).
Widmoser, P. A discussion on and alternative to the Penman–Monteith equation. Agric. Water Manag. 96, 711–721 (2009).
Jones, H. G. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology (Cambridge Univ. Press, 2014).
Gates, D. M. Biophysical Ecology (Springer-Verlag, 1980).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2014).
Campbell, G. S. & Norman, J. M. An Introduction to Environmental Biophysics (Springer, 1998).
Bergman, T. L., Lavine, A. S., Incropera, F. P. & DeWitt, D. P. Fundamentals of Heat and Mass Transfer 7th edn (John Wiley, 2011).
Goff, J. A. & Gratch, S. Low-pressure properties of water from −160 to 212 °F. Trans. Am. Soc. Heat. Vent. Eng. 51, 125–164 (1946).
Michaletz, S. T. & Johnson, E. A. Foliage influences forced convection heat transfer in conifer branches and buds. New Phytol. 170, 87–98 (2006).
Acknowledgements
This paper is dedicated to the memory of Dr David M. Gates, who began studying the thermoregulation and energy budgets of leaves more than a half century ago. The authors thank B. Blonder for providing thoughtful comments on an earlier version of the paper, L. Stockton for helping collect leaf trait and gas exchange data, H. Adams, A. Collins, T. Dickman, C. Grossiord, A. Henderson, J. Reithel and S. Sevanto for providing meteorological and phenological data, and J. Finch and J. Draper for supplying the Brachypodium distachyon image used in Fig. 1. S.T.M. was supported by a Director's Fellowship from the Los Alamos National Laboratory. S.T.M., M.D.W., J.Z., M.K. and B.J.E. were supported by NSF MacroSystems award 1065861. B.R.H. was supported under NSF award IOS-0950998 and NSF MacroSystems award 1241873. N.G.M. was supported by SUMO and NGEE-Tropics support from the Department of Energy, Office of Science. B.J.E. was supported by a fellowship from the Aspen Center for Environmental Studies.
Author information
Authors and Affiliations
Contributions
S.T.M., M.D.W., N.G.M., J.Z., M.K., B.R.H. and B.J.E. compiled data, developed theory, performed analyses and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Additional theory, Supplementary references, Supplementary Figs 1-4 and Supplementary Tables 1-3. (PDF 786 kb)
Rights and permissions
About this article
Cite this article
Michaletz, S., Weiser, M., McDowell, N. et al. The energetic and carbon economic origins of leaf thermoregulation. Nature Plants 2, 16129 (2016). https://doi.org/10.1038/nplants.2016.129
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2016.129
This article is cited by
-
Temperature dependence of carbon metabolism in the leaves in sun and shade in a subtropical forest
Oecologia (2024)
-
Geographical variation in functional traits of leaves of Caryopteris mongholica and the role of climate
BMC Plant Biology (2023)
-
Using spatially variable nitrogen application and crop responses to evaluate crop nitrogen use efficiency
Nutrient Cycling in Agroecosystems (2023)
-
Diurnal variation in rectal and cutaneous temperatures of horses housed under different management conditions
International Journal of Biometeorology (2022)
-
A compendium of vivipary in the Cactaceae: new reports, data, and research prospects
Brazilian Journal of Botany (2022)