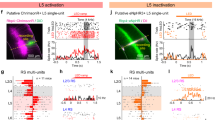
Abstract
Neurons in the recipient layers of sensory cortices receive excitatory input from two major sources: the feedforward thalamocortical and recurrent intracortical inputs. To address their respective functional roles, we developed a new method for silencing cortex by competitively activating GABAA while blocking GABAB receptors. In the rat primary auditory cortex, in vivo whole-cell recording from the same neuron before and after local cortical silencing revealed that thalamic input occupied the same area of frequency-intensity tonal receptive field as the total excitatory input, but showed a flattened tuning curve. In contrast, excitatory intracortical input was sharply tuned with a tuning curve that closely matched that of suprathreshold responses. This can be attributed to a selective amplification of cortical cells' responses at preferred frequencies by intracortical inputs from similarly tuned neurons. Thus, weakly tuned thalamocortical inputs determine the subthreshold responding range, whereas intracortical inputs largely define the tuning. Such circuits may ensure a faithful conveyance of sensory information.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Reid, R.C. & Alonso, J.M. Specificity of monosynaptic connections from thalamus to visual cortex. Nature 378, 281–284 (1995).
Ferster, D., Chung, S. & Wheat, H. Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature 380, 249–252 (1996).
Chung, S. & Ferster, D. Strength and orientation tuning of the thalamic input to simple cells revealed by electrically evoked cortical suppression. Neuron 20, 1177–1189 (1998).
Miller, L.M., Escabi, M.A., Read, H.L. & Schreiner, C.E. Functional convergence of response properties in the auditory thalamocortical system. Neuron 32, 151–160 (2001).
Bruno, R.M. & Sakmann, B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science 312, 1622–1627 (2006).
Douglas, R.J., Koch, C., Mahowald, M., Martin, K.A. & Suarez, H.H. Recurrent excitation in neocortical circuits. Science 269, 981–985 (1995).
Somers, D.C., Nelson, S.B. & Sur, M. An emergent model of orientation selectivity in cat visual cortical simple cells. J. Neurosci. 15, 5448–5465 (1995).
Miller, K.D., Pinto, D.J. & Simons, D.J. Processing in layer 4 of the neocortical circuit: new insights from visual and somatosensory cortex. Curr. Opin. Neurobiol. 11, 488–497 (2001).
Alonso, J.M. & Swadlow, H.A. Thalamocortical specificity and the synthesis of sensory cortical receptive fields. J. Neurophysiol. 94, 26–32 (2005).
Miller, L.M., Escabi, M.A., Read, H.L. & Schreiner, C.E. Spectrotemporal receptive fields in the lemniscal auditory thalamus and cortex. J. Neurophysiol. 87, 516–527 (2002).
Miller, L.M., Escabi, M.A. & Schreiner, C.E. Feature selectivity and interneuronal cooperation in the thalamocortical system. J. Neurosci. 21, 8136–8144 (2001).
Martinez, L.M. et al. Receptive field structure varies with layer in the primary visual cortex. Nat. Neurosci. 8, 372–379 (2005).
Fox, K., Wright, N., Wallace, H. & Glazewski, S. The origin of cortical surround receptive fields studied in the barrel cortex. J. Neurosci. 23, 8380–8391 (2003).
Kaur, S., Lazar, R. & Metherate, R. Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J. Neurophysiol. 91, 2551–2567 (2004).
Zhang, Y. & Suga, N. Corticofugal amplification of subcortical responses to single tone stimuli in the mustached bat. J. Neurophysiol. 78, 3489–3492 (1997).
Chung, S. & Ferster, D. Strength and orientation tuning of the thalamic input to simple cells revealed by electrically evoked cortical suppression. Neuron 20, 1177–1189 (1998).
Volgushev, M., Vidyasagar, T.R., Chistiakova, M., Yousef, T. & Eysel, U.T. Membrane properties and spike generation in rat visual cortical cells during reversible cooling. J. Physiol. (Lond.) 522, 59–76 (2000).
Villa, A.E. et al. Corticofugal modulation of the information processing in the auditory thalamus of the cat. Exp. Brain Res. 86, 506–517 (1991).
Yamauchi, T., Hori, T. & Takahashi, T. Presynaptic inhibition by muscimol through GABAB receptors. Eur. J. Neurosci. 12, 3433–3436 (2000).
Porter, J.T. & Nieves, D. Presynaptic GABAB receptors modulate thalamic excitation of inhibitory and excitatory neurons in the mouse barrel cortex. J. Neurophysiol. 92, 2762–2770 (2004).
Roerig, B. & Chen, B. Relationships of local inhibitory and excitatory circuits to orientation-preference maps in ferret visual cortex. Cereb. Cortex 12, 187–198 (2002).
Marino, J. et al. Invariant computations in local cortical networks with balanced excitation and inhibition. Nat. Neurosci. 8, 194–201 (2005).
Zhang, L.I., Bao, S. & Merzenich, M.M. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat. Neurosci. 4, 1123–1130 (2001).
Bringuier, V., Chavane, F., Glaeser, L. & Fregnac, Y. Horizontal propagation of visual activity in the synaptic integration field of area 17 neurons. Science 283, 695–699 (1999).
Zhang, L.I., Tan, A.Y., Schreiner, C.E. & Merzenich, M.M. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature 424, 201–205 (2003).
Wehr, M. & Zador, A.M. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426, 442–446 (2003).
Tan, A.Y., Zhang, L.I., Merzenich, M.M. & Schreiner, C.E. Tone-evoked excitatory and inhibitory synaptic conductances of primary auditory cortex neurons. J. Neurophysiol. 92, 630–643 (2004).
Carandini, M. & Ferster, D. Membrane potential and firing rate in cat primary visual cortex. J. Neurosci. 20, 470–484 (2000).
Anderson, J.S., Carandini, M. & Ferster, D. Orientation tuning of input conductance, excitation and inhibition in cat primary visual cortex. J. Neurophysiol. 84, 909–926 (2000).
Priebe, N. & Ferster, D. Direction selectivity of excitation and inhibition in simple cells of the cat primary visual cortex. Neuron 45, 133–145 (2005).
Wilent, W.B. & Contreras, D. Stimulus-dependent changes in spike threshold enhance feature selectivity in rat barrel cortex neurons. J. Neurosci. 25, 2983–2991 (2005).
Swadlow, H.A. & Gusev, A.G. Receptive-field construction in cortical inhibitory interneurons. Nat. Neurosci. 5, 403–404 (2002).
Wu, G.K., Li, P., Tao, H.W. & Zhang, L.I. Nonmonotonic synaptic excitation and imbalanced inhibition underlying cortical intensity tuning. Neuron 52, 705–715 (2006).
Winer, J.A., Miller, L.M., Lee, C.C. & Schreiner, C.E. Auditory thalamocortical transformation: structure and function. Trends Neurosci. 28, 255–263 (2005).
Calford, M.B. & Webster, W.R. Auditory representation within principal division of cat medial geniculate body: an electrophysiology study. J. Neurophysiol. 45, 1013–1028 (1981).
Moore, C.I. & Nelson, S.B. Spatio-temporal subthreshold receptive fields in the vibrissa representation of rat primary somatosensory cortex. J. Neurophysiol. 80, 2882–2892 (1998).
Margrie, T.W., Brecht, M. & Sakmann, B. In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflugers Arch. 444, 491–498 (2002).
Games, K.D. & Winer, J.A. Layer V in rat auditory cortex: projections to the inferior colliculus and contralateral cortex. Hear. Res. 34, 1–25 (1988).
Horikawa, K. & Armstrong, W.E. A versatile means of intracellular labelling: injection of biocytin and its detection with avidin conjugates. J. Neurosci. Methods 25, 1–11 (1988).
Zhu, Y., Stornetta, R.L. & Zhu, J.J. Chandelier cells control excessive cortical excitation: characteristics of whisker-evoked synaptic responses of layer 2/3 nonpyramidal and pyramidal neurons. J. Neurosci. 24, 5101–5108 (2004).
Hirsch, J.A., Alonso, J.M., Reid, R.C. & Martinez, L.M. Synaptic integration in striate cortical simple cells. J. Neurosci. 18, 9517–9528 (1998).
Borg-Graham, L.J., Monier, C. & Fregnac, Y. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature 393, 369–373 (1998).
Acknowledgements
HThis work was supported by grants to L.I.Z. from the US National Institutes of Health/National Institute on Deafness and Other Communication Disorders, the Searle Scholar Program, the Esther A. & and Joseph Klingenstein Fund, Inc., and the David and Lucile Packard Foundation.
Author information
Authors and Affiliations
Contributions
L.I.Z. conceived the study. G.K.W. and B.L. carried out the in vivo experiments and data analysis. B.L. modeled the effects of cocktail application on synaptic responses. R.A. was involved in current-clamp recordings. L.I.Z. and H.W.T. supervised the project and wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Table 1 and Notes 1 and 2. (PDF 421 kb)
Rights and permissions
About this article
Cite this article
Liu, Bh., Wu, G., Arbuckle, R. et al. Defining cortical frequency tuning with recurrent excitatory circuitry. Nat Neurosci 10, 1594–1600 (2007). https://doi.org/10.1038/nn2012
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn2012
This article is cited by
-
The extracellular matrix regulates cortical layer dynamics and cross-columnar frequency integration in the auditory cortex
Communications Biology (2021)
-
Corticostriatal control of defense behavior in mice induced by auditory looming cues
Nature Communications (2021)
-
Optogenetic stimulation of the VTA modulates a frequency-specific gain of thalamocortical inputs in infragranular layers of the auditory cortex
Scientific Reports (2019)
-
Progress and challenges for understanding the function of cortical microcircuits in auditory processing
Nature Communications (2017)
-
Lateral geniculate neurons projecting to primary visual cortex show ocular dominance plasticity in adult mice
Nature Neuroscience (2017)