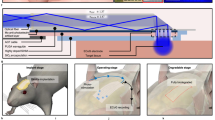
Abstract
Bioresorbable silicon electronics technology offers unprecedented opportunities to deploy advanced implantable monitoring systems that eliminate risks, cost and discomfort associated with surgical extraction. Applications include postoperative monitoring and transient physiologic recording after percutaneous or minimally invasive placement of vascular, cardiac, orthopaedic, neural or other devices. We present an embodiment of these materials in both passive and actively addressed arrays of bioresorbable silicon electrodes with multiplexing capabilities, which record in vivo electrophysiological signals from the cortical surface and the subgaleal space. The devices detect normal physiologic and epileptiform activity, both in acute and chronic recordings. Comparative studies show sensor performance comparable to standard clinical systems and reduced tissue reactivity relative to conventional clinical electrocorticography (ECoG) electrodes. This technology offers general applicability in neural interfaces, with additional potential utility in treatment of disorders where transient monitoring and modulation of physiologic function, implant integrity and tissue recovery or regeneration are required.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Niedermeyer, E. & da Silva, F. L. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields (Lippincott Williams Wilkins, 2005).
Stacey, W. C. & Litt, B. Technology insight: neuroengineering and epilepsy—designing devices for seizure control. Nature Clin. Pract. Neurol. 4, 190–201 (2008).
McKhann, G. M., Schoenfeld-McNeill, J., Born, D. E., Haglund, M. M. & Ojemann, G. A. Intraoperative hippocampal electrocorticography to predict the extent of hippocampal resection in temporal lobe epilepsy surgery. J. Neurosurg. 93, 44–52 (2000).
Whitmer, D. et al. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease. Front. Hum. Neurosci. 6, 155 (2012).
Litt, B. et al. Epileptic seizures may begin hours in advance of clinical onset: a report of five patients. Neuron 30, 51–64 (2001).
Shapiro, M., Becske, T., Sahlein, D., Babb, J. & Nelson, P. K. Stent-supported aneurysm coiling: a literature survey of treatment and follow-up. Am. J. Neuroradiol. 33, 159–163 (2012).
Wholey, M. H. et al. Global experience in cervical carotid artery stent placement. Catheter. Cardio. Inter. 50, 160–167 (2000).
Frizzel, R. T. & Fisher, W. S. III Cure, morbidity, and mortality associated with embolization of brain arteriovenous malformations: a review of 1246 patients in 32 series over a 35-year period. Neurosurgery 37, 1031–1040 (1995).
McNett, M. M. & Horowitz, D. A. International multidisciplinary consensus conference on multimodality monitoring: ICU processes of care. Neurocrit. Care 21, 215–228 (2014).
Mayevsky, A., Manor, T., Meilin, S., Doron, A. & Ouaknine, G. E. Real-time multiparametric monitoring of the injured human cerebral cortex–a new approach. Acta Neurochir. Suppl. 71, 78–81 (1998).
Khodagholy, D. et al. In vivo recordings of brain activity using organic transistors. Nature Commun. 4, 1575 (2013).
Viventi, J. et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nature Neurosci. 14, 1599–1605 (2011).
Khodagholy, D. et al. NeuroGrid: recording action potentials from the surface of the brain. Nature Neurosci. 18, 310–315 (2015).
Escabí, M. A. et al. A high-density, high-channel count, multiplexed μECoG array for auditory-cortex recordings. J. Neurophysiol. 112, 1566–1583 (2014).
Qing, Q. et al. Nanowire transistor arrays for mapping neural circuits in acute brain slices. Proc. Natl Acad. Sci. USA 107, 1882–1887 (2010).
Xiang, Z. et al. Ultra-thin flexible polyimide neural probe embedded in a dissolvable maltose-coated microneedle. J. Micromech. Microeng. 24, 065015 (2014).
Tian, B. et al. Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science 329, 830–834 (2010).
Kozai, T. D. Y. et al. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nature Mater. 11, 1065–1073 (2012).
Kuzum, D. et al. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nature Commun. 5, 5259 (2014).
Vitale, F., Summerson, S. R., Aazhang, B., Kemere, C. & Pasquali, M. Neural stimulation and recording with bidirectional, soft carbon nanotube fiber microelectrodes. ACS Nano 9, 4465–4474 (2015).
Daube, J. & Rubin, D. Clinical Neurophysiology (Oxford Univ. Press, 2009).
King-Stephens, D. et al. Lateralization of mesial temporal lobe epilepsy with chronic ambulatory electrocorticography. Epilepsia 56, 959–967 (2015).
Kang, S.-K. et al. Bioresorbable silicon electronic sensors for the brain. Nature 530, 71–76 (2016).
Saha, R. et al. Highly doped polycrystalline silicon microelectrodes reduce noise in neuronal recordings in vivo. IEEE Trans. Neural. Sys. Rehab. Eng. 18, 489–497 (2010).
Fontes, M. B. A. Electrodes for bio-application: recording and stimulation. J. Phys. Conf. Ser. 421, 012019 (2013).
Oskam, G., Long, J. G., Natarajan, A. & Searson, P. C. Electrochemical deposition of metals onto silicon. J. Phys. D 31, 1927–1949 (1998).
Zhang, X. G. Electrochemistry of Silicon and its Oxide (Kluwer Academic, 2001).
Schmickler, W. & Santos, E. Interfacial Electrochemistry Ch. 11 (Springer, 2010).
Morita, M., Ohmi, T., Hasegawa, E., Kawakami, M. & Ohwada, M. Growth of native oxide on a silicon surface. J. Appl. Phys. 68, 1272–1281 (1990).
Seidel, H., Csepregi, L., Heuberger, A. & Baumgartel, H. Anisotropic etching of crystalline silicon in alkaline solutions: I. Orientation dependence and behavior of passivation layers. J. Electrochem. Soc. 137, 3612–3626 (1990).
Gentile, P., Chiono, V., Carmagnola, I. & Hatton, P. V. An overview of poly(lactic-coglycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 15, 3640–3659 (2014).
Shaw, F.-Z. Is spontaneous high-voltage rhythmic spike discharge in Long Evans rats an absence-like seizure activity? J. Neurophysiol. 91, 63–77 (2004).
Pearce, P. S. et al. Spike–wave discharges in adult Sprague–Dawley rats and their implications for animal models of temporal lobe epilepsy. Epilepsy Behav. 32, 121–131 (2014).
Rodgers, K. M., Dudek, F. E. & Barth, D. S. Progressive, seizure-like, spike-wave discharges are common in both injured and uninjured sprague-dawley rats: implications for the fluid percussion injury model of post-traumatic epilepsy. J. Neurosci. 35, 9194–9204 (2015).
Polikov, V. S., Tresco, P. A. & Reichert, W. M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 148, 1–18 (2005).
Ryu, S. I. & Shenoy, K. V. Human cortical prostheses: lost in translation? Neurosurg. Focus 27, E5 (2009).
Biran, R., Martin, D. C. & Tresco, P. A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 195, 115–126 (2005).
Biran, R., Martin, D. C. & Tresco, P. A. The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. J. Biomed. Mater. Res. A 82, 169–178 (2007).
Hwang, S.-W. et al. A physically transient form of silicon electronics. Science 337, 1640–1644 (2012).
Yin, L. et al. Dissolvable metals for transient electronics. Adv. Funct. Mater. 24, 645–658 (2014).
Badawy, W. A. & Al-Kharafi, F. M. Corrosion and passivation behaviors of molybdenum in aqueous solutions of different pH. Electrochim. Acta 44, 693–702 (1998).
Kang, S. et al. Biodegradable thin metal foils and spin-on glass materials for transient electronics. Adv. Funct. Mater. 7, 9297–9305 (2015).
Kang, S.-K. et al. Dissolution behaviors and applications of silicon oxides and nitrides in transient electronics. Adv. Funct. Mater. 24, 4427–4434 (2014).
Hwang, S.-W. et al. Dissolution chemistry and biocompatibility of single-crystalline silicon nanomembranes and associated materials for transient electronics. ACS Nano 8, 5843–5851 (2014).
Kue, R. et al. Enhanced proliferation and osteocalcin production by human osteoblast-like MG63 cells on silicon nitride ceramic discs. Biomaterials 20, 1195–1201 (1999).
Bal, B. S. & Rahaman, M. N. Orthopedic applications of silicon nitride ceramics. Acta Biomater. 8, 2889–2898 (2012).
Acknowledgements
The work was funded by the Defense Advanced Research Projects Agency, the Penn Medicine Neuroscience Center Pilot Grant, T32- Brain Injury Research Training Grant (5T32NS043126-12) and the Mirowski Family Foundation. Images in figures 2e, 3a and 6d from 3D Rat Anatomy Software (www.biosphera.org).
Author information
Authors and Affiliations
Contributions
K.J.Y., D.K., B.L. and J.A.R. designed the research. K.J.Y., D.K., S.-W.H., B.H.K., N.H.K., S.M.W., K.C., H.F., K.J.S., H.N.L., S.-K.K., J.-H.K. and J.Y.L. fabricated the devices and electronics. K.J.Y., D.K., S.M.W., M. Trumpis, H.F., M. Thompson, H.B., M.A.D., T.L. and J.V. conceived and performed bench tests, and analysis. D.K., K.J.Y., H.J., A.G.R., M.A.D. and T.H.L. performed in vivo experiments and analysed the data. D.T. and F.E.J. performed biocompatibility and histology studies. H.C. and Y.H. performed mechanical simulations. K.J.Y., D.K., A.G.R., M.Trumpis, J.V., B.L. and J.A.R. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 7145 kb)
Supplementary Information
Supplementary movie 1 (MOV 16023 kb)
Supplementary Information
Supplementary movie 2 (MOV 1642 kb)
Supplementary Information
Supplementary movie 3 (MOV 1383 kb)
Supplementary Information
Supplementary movie 4 (MOV 1413 kb)
Supplementary Information
Supplementary movie 5 (MOV 1455 kb)
Supplementary Information
Supplementary movie 6 (MOV 4949 kb)
Rights and permissions
About this article
Cite this article
Yu, K., Kuzum, D., Hwang, SW. et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nature Mater 15, 782–791 (2016). https://doi.org/10.1038/nmat4624
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4624
This article is cited by
-
Fully bioresorbable hybrid opto-electronic neural implant system for simultaneous electrophysiological recording and optogenetic stimulation
Nature Communications (2024)
-
Advances in Wireless, Batteryless, Implantable Electronics for Real-Time, Continuous Physiological Monitoring
Nano-Micro Letters (2024)
-
Hybrid graphene electrode for the diagnosis and treatment of epilepsy in free-moving animal models
NPG Asia Materials (2023)
-
Tracking neural activity from the same cells during the entire adult life of mice
Nature Neuroscience (2023)
-
A soft, high-density neuroelectronic array
npj Flexible Electronics (2023)