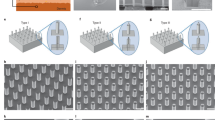
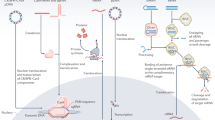
Abstract
The controlled delivery of nucleic acids to selected tissues remains an inefficient process mired by low transfection efficacy, poor scalability because of varying efficiency with cell type and location, and questionable safety as a result of toxicity issues arising from the typical materials and procedures employed. High efficiency and minimal toxicity in vitro has been shown for intracellular delivery of nuclei acids by using nanoneedles, yet extending these characteristics to in vivo delivery has been difficult, as current interfacing strategies rely on complex equipment or active cell internalization through prolonged interfacing. Here, we show that a tunable array of biodegradable nanoneedles fabricated by metal-assisted chemical etching of silicon can access the cytosol to co-deliver DNA and siRNA with an efficiency greater than 90%, and that in vivo the nanoneedles transfect the VEGF-165 gene, inducing sustained neovascularization and a localized sixfold increase in blood perfusion in a target region of the muscle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Leader, B., Baca, Q. J. & Golan, D. E. Protein therapeutics: A summary and pharmacological classification. Nature Rev. Drug Discov. 7, 21–39 (2008).
Vlieghe, P., Lisowski, V., Martinez, J. & Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 15, 40–56 (2010).
Kole, R., Krainer, A. R. & Altman, S. RNA therapeutics: Beyond RNA interference and antisense oligonucleotides. Nature Rev. Drug Discov. 11, 125–140 (2012).
Khademhosseini, A. Microscale technologies for tissue engineering and biology. Proc. Natl Acad. Sci. USA 103, 2480–2487 (2006).
Whitehead, K. A., Langer, R. & Anderson, D. G. Knocking down barriers: Advances in siRNA delivery. Nature Rev. Drug Discov. 8, 129–138 (2009).
Saltzman, W. M. & Luo, D. Synthetic DNA delivery systems. Nature Biotechnol. 18, 33–37 (2000).
Thomas, C. E., Ehrhardt, A. & Kay, M. A. Progress and problems with the use of viral vectors for gene therapy. Nature Rev. Genet. 4, 346–358 (2003).
Mingozzi, F. & High, K. A. Therapeutic in vivo gene transfer for genetic disease using AAV: Progress and challenges. Nature Rev. Genet. 12, 341–355 (2011).
Lefesvre, P., Attema, J. & van Bekkum, D. A comparison of efficacy and toxicity between electroporation and adenoviral gene transfer. BMC Mol. Biol. 3, 12 (2002).
Aihara, H. & Miyazaki, J-I. Gene transfer into muscle by electroporation in vivo. Nature Biotechnol. 16, 867–870 (1998).
Nishikawa, M. & Huang, L. Nonviral vectors in the new millennium: Delivery barriers in gene transfer. Hum. Gene Ther. 12, 861–870 (2001).
Shalek, A. K. et al. Vertical silicon nanowires as a universal platform for delivering biomolecules into living cells. Proc. Natl Acad. Sci. USA 107, 1870–1875 (2010).
Robinson, J. T. et al. Vertical nanowire electrode arrays as a scalable platform for intracellular interfacing to neuronal circuits. Nature Nanotech. 7, 180–184 (2012).
Yan, R. et al. Nanowire-based single-cell endoscopy. Nature Nanotech. 7, 191–196 (2011).
Han, S. W. et al. High-efficiency DNA injection into a single human mesenchymal stem cell using a nanoneedle and atomic force microscopy. Nanomedicine: Nanotechnol. Biol. Med. 4, 215–225 (2008).
Shalek, A. K. et al. Nanowire-mediated delivery enables functional interrogation of primary immune cells: Application to the analysis of chronic lymphocytic leukemia. Nano Lett. 12, 6498–6504 (2012).
Xu, A. M. et al. Quantification of nanowire penetration into living cells. Nature Commun. 5, 3613 (2014).
Sharei, A. et al. A vector-free microfluidic platform for intracellular delivery. Proc. Natl Acad. Sci. USA 110, 2082–2087 (2013).
Hanson, L., Lin, Z. C., Xie, C., Cui, Y. & Cui, B. Characterization of the cell–nanopillar interface by transmission electron microscopy. Nano Lett. 12, 5815–5820 (2012).
Xie, X. et al. Mechanical model of vertical nanowire cell penetration. Nano Lett. 13, 6002–6008 (2013).
Xie, C., Lin, Z., Hanson, L., Cui, Y. & Cui, B. Intracellular recording of action potentials by nanopillar electroporation. Nature Nanotech. 7, 185–190 (2012).
Wang, Y. et al. Poking cells for efficient vector-free intracellular delivery. Nature Commun. 5, 4466 (2014).
Anderson, S. H. C., Elliott, H., Wallis, D. J., Canham, L. T. & Powell, J. J. Dissolution of different forms of partially porous silicon wafers under simulated physiological conditions. Phys. Status Solidi A 197, 331–335 (2003).
Canham, L. T. Bioactive silicon structure fabrication through nanoetching techniques. Adv. Mater. 7, 1033–1037 (1995).
Chiappini, C. et al. Tailored porous silicon microparticles: Fabrication and properties. ChemPhysChem 11, 1029–1035 (2010).
Tasciotti, E. et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nature Nanotech. 3, 151–157 (2008).
Tanaka, T. et al. Sustained small interfering RNA delivery by mesoporous silicon particles. Cancer Res. 70, 3687–3696 (2010).
Park, J-H. et al. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nature Mater. 8, 331–336 (2009).
Goh, A. S-W. et al. A novel approach to brachytherapy in hepatocellular carcinoma using a phosphorous32 (32P) brachytherapy delivery device—a first-in-man study. Int. J. Radiat. Oncol. Biol. Phys. 67, 786–792 (2007).
Anglin, E., Cheng, L., Freeman, W. & Sailor, M. Porous silicon in drug delivery devices and materials. Adv. Drug Deliv. Rev. 60, 1266–1277 (2008).
Chiappini, C., Liu, X., Fakhoury, J. R. & Ferrari, M. Biodegradable porous silicon barcode nanowires with defined geometry. Adv. Funct. Mater. 20, 2231–2239 (2010).
Obataya, I., Nakamura, C., Han, S., Nakamura, N. & Miyake, J. Mechanical sensing of the penetration of various nanoneedles into a living cell using atomic force microscopy. Biosens. Bioelectron. 20, 1652–1655 (2005).
Na, Y-R. et al. Probing enzymatic activity inside living cells using a nanowire–cell ‘sandwich’ assay. Nano Lett. 13, 153–158 (2013).
Muralidharan-Chari, V., Clancy, J. W., Sedgwick, A. & D’Souza-Schorey, C. Microvesicles: Mediators of extracellular communication during cancer progression. J. Cell Sci. 123, 1603–1611 (2010).
Ratajczak, J. et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20, 847–856 (2006).
Yuan, A. et al. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS ONE 4, e4722 (2009).
Shen, Z. et al. Porous silicon as a versatile platform for laser desorption/ionization mass spectrometry. Anal. Chem. 73, 612–619 (2000).
Lee, J. W., Park, J-H. & Prausnitz, M. R. Dissolving microneedles for transdermal drug delivery. Biomaterials 29, 2113–2124 (2008).
Gross, S. et al. Bioluminescence imaging of myeloperoxidase activity in vivo. Nature Med. 15, 455–461 (2009).
Luxembourg, A., Evans, C. F. & Hannaman, D. Electroporation-based DNA immunisation: Translation to the clinic. Expert Opin. Biol. Ther. 7, 1647–1664 (2007).
Yancopoulos, G. D. et al. Vascular-specific growth factors and blood vessel formation. Nature 407, 242–248 (2000).
Tammela, T., Enholm, B., Alitalo, K. & Paavonen, K. The biology of vascular endothelial growth factors. Cardiovasc. Res. 65, 550–563 (2005).
DeMuth, P. C. et al. Polymer multilayer tattooing for enhanced DNA vaccination. Nature Mater. 12, 367–376 (2013).
Kim, Y-C., Park, J-H. & Prausnitz, M. R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 64, 1547–1568 (2012).
Gershonowitz, A. & Gat, A. VoluDerm Microneedle Technology for Skin Treatments—In Vivo Histological Evidence. J. Cosmetic Laser Surg. 17, 9–14 (2015).
Barrientos, S., Stojadinovic, O., Golinko, M. S., Brem, H. & Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 16, 585–601 (2008).
Acknowledgements
The authors would like to thank M. Giacca at the International Centre for Genetic Engineering and Biotechnology (ICGEB in Trieste, Italy) for kindly donating the pDNA-expressing VEGF-165, and I. K. Yazdi and R. Palomba for the amplification and purification of the plasmids used in the study. We are grateful to S. Amra for histology-slide preparation, D. Tinkey for surgical procedures, S. Zacchigna for suggestions on PCR protocols, P. Campagnolo for advice on SMA and isolectin staining, and S. T. Gammon for advice on bioluminescence imaging with luminol. This work was financially supported by: the US Department of Defense (W81XWH-12-10414) and the NIH (1R21CA173579-01A1 and 5U54CA143837); C.C. was supported by a Newton International Fellowship and a Marie Curie International Incoming Fellowship; J.O.M. was supported by a NIH pre-doctoral fellowship 5F31CA154119-02. M.M.S. holds an ERC grant ‘Naturale-CG’ and is supported by a Wellcome Trust Senior Investigator Award. E.T. holds grants from the Hearst Foundation and the Cullen Trust Foundation. C.C. and M.M.S. thank the Rosetrees Trust and the Stoneygate Trust for funding.
Author information
Authors and Affiliations
Contributions
C.C., E.T. and M.M.S. designed the research. C.C. and E.T. conceived the nanoneedles; C.C. developed the nanoneedles with contributions from X.L.; C.C. evaluated loading, release, delivery and efficacy with contributions from E.D.R.; C.C. imaged biodegradation and cell interaction with contributions from E.D.R.; C.C. wrote the initial manuscript with contributions from E.D.R. and J.O.M. C.C. performed all electron microscopy analysis. E.D.R. and J.O.M. assessed cytocompatibility; E.D.R. designed and performed animal surgeries, intravital imaging and quantification of vascularization, vasculature pattern, and extravasation and flow rate over time on VEGF treatment, with contributions from J.O.M. J.O.M. evaluated degradation by ICP-AES, fluorescent and bioluminescent imaging and analysis, real-time PCR, and histological evaluation and staining of tissues with contributions from E.D.R. J.S. performed compressive mechanical testing. E.T. and M.M.S. contributed equally to the work, with M.M.S. supervising the in vitro studies and E.T. the in vivo work. All authors discussed and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1353 kb)
Supplementary Movie 1
Supplementary Movie 1 (MOV 14352 kb)
Supplementary Movie 2
Supplementary Movie 2 (MOV 2914 kb)
Supplementary Movie 3
Supplementary Movie 3 (MOV 30904 kb)
Rights and permissions
About this article
Cite this article
Chiappini, C., De Rosa, E., Martinez, J. et al. Biodegradable silicon nanoneedles delivering nucleic acids intracellularly induce localized in vivo neovascularization. Nature Mater 14, 532–539 (2015). https://doi.org/10.1038/nmat4249
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4249
This article is cited by
-
Electroactive nanoinjection platform for intracellular delivery and gene silencing
Journal of Nanobiotechnology (2023)
-
Nanotechnology in stem cell research and therapy
Journal of Nanoparticle Research (2023)
-
Role of actin cytoskeleton in cargo delivery mediated by vertically aligned silicon nanotubes
Journal of Nanobiotechnology (2022)
-
Recent advances in developing active targeting and multi-functional drug delivery systems via bioorthogonal chemistry
Signal Transduction and Targeted Therapy (2022)
-
Semi-Implantable Bioelectronics
Nano-Micro Letters (2022)