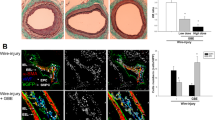
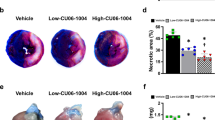
Abstract
Carbon monoxide (CO), one of the products of heme oxygenase action on heme, prevents arteriosclerotic lesions that occur following aorta transplantation; pre-exposure to 250 parts per million of CO for 1 hour before injury suppresses stenosis after carotid balloon injury in rats as well as in mice. The protective effect of CO is associated with a profound inhibition of graft leukocyte infiltration/activation as well as with inhibition of smooth muscle cell proliferation. The anti-proliferative effect of CO in vitro requires the activation of guanylate cyclase, the generation of cGMP, the activation of p38 mitogen-activated protein kinases and the expression of the cell cycle inhibitor p21Cip1. These findings demonstrate a protective role for CO in vascular injury and support its use as a therapeutic agent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Berk, B.C. Vascular smooth muscle growth: autocrine growth mechanisms. Physiol. Rev. 81, 999–1030 (2001).
Libby, P. & Pober, J.S. Chronic rejection. Immunity 14, 387–397 (2001).
Murakami, S. et al. Heat shock protein (HSP) 47 and collagen are upregulated during neointimal formation in the balloon-injured rat carotid artery. Atherosclerosis 157, 361–368 (2001).
Petkova, V. et al. Interaction between YY1 and the retinoblastoma protein. Regulation of cell cycle progression in differentiated cells. J. Biol. Chem. 276, 7932–7936 (2001).
DeGregori, J., Leone, G., Miron, A., Jakoi, L. & Nevins, J.R. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc. Natl. Acad. Sci. USA 94, 7245–7250 (1997).
Toyoshima, H. & Hunter, T. Protein p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 78, 67–74 (1994).
Luo, Y., Hurwitz, J. & Massague, J. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature 375, 159–161 (1995).
Yang, Z.Y. et al. Role of the p21 cyclin-dependent kinase inhibitor in limiting intimal cell proliferation in response to arterial injury. Proc. Natl. Acad. Sci. USA 93, 7905–7910 (1996).
Bach, F.H. et al. Accommodation of vascularized xenografts:expression of “protective genes” by donor endothelial cells in a host Th2 cytokine environment. Nature Med. 3, 196–202 (1997).
Soares, M.P. et al. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nature Med. 4, 1073–1077 (1998).
Tenhunen, R., Marver, H.S. & Schmid, R. The enzymatic conversion of heme tobilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. USA 61, 748–755 (1968).
Sato, K. et al. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J. Immunol. 166, 4185–4194 (2001).
Otterbein, L.E. et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nature Med. 6, 422–428 (2000).
Fujita, T. et al. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nature Med. 7, 598–604 (2001).
Brouard, S. et al. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J. Exp. Med. 192, 1015–1026 (2000).
Petrache, I., Otterbein, L.E., Alam, J., Wiegand, G.W. & Choi, A.M. Heme oxygenase-1 inhibits TNF-α-induced apoptosis in cultured fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 278, L312–L319 (2000).
Otterbein, L.E., Mantell, L.L. & Choi, A.M. Carbon monoxide provides protection against hyperoxic lung injury. Am. J. Physiol. 276, L688–L694 (1999).
Plissonnier, D. et al. Sequential immunological targeting of chronic experimental arterial allograft. Transplantation 60, 414–424 (1995).
Shimizu, K. et al. Host bone-marrow cells are a source of donor intimal smooth- muscle-like cells in murine aortic transplant arteriopathy. Nature Med. 7, 738–741 (2001).
Indolfi, C. et al. Smooth muscle cell proliferation is proportional to the degree of balloon injury in a rat model of angioplasty. Circulation 92, 1230–1235 (1995).
Peyton, K.J. et al. Heme oxygenase-1-derived carbon monoxide is an autocrine inhibitor of vascular SMC growth. Blood 99, 4443–4448 (2002).
Duckers, H.J. et al. Heme oxygenase-1 protects against vascular constriction and proliferation. Nature Med. 7, 693–698 (2001).
Tang, H.Y. et al. Constitutive expression of the cyclin-dependent kinase inhibitor p21 is transcriptionally regulated by the tumor suppressor protein p53. J. Biol. Chem. 273, 29156–29163 (1998).
Cuenda, A. et al. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 364, 229–233 (1995).
Otterbein, L.E. & Choi, A.M. Heme oxygenase: colors of defense against cellular stress. Am. J. Physiol. Lung Cell Mol. Physiol. 279, L1029–L1037 (2000).
Ishikawa, K., Navab, M., Leitinger, N., Fogelman, A.M. & Lusis, A.J. Induction of hemeoxygenase-1 inhibits the monocyte transmigration induced by mildly oxidized LDL. J. Clin. Invest. 100, 1209–1216 (1997).
Lee, T.S. & Chau, L.Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nature Med. 8, 240–246 (2002).
Coceani, F. Carbon monoxide and dilation of blood vessels. Science 260, 739 (1993).
Naseem, K.M. & Bruckdorfer, K.R. Inhibition of platelet activation by carbon monoxide: comparison with nitric oxide. Biochem. Soc. Trans. 3, 396S (1997).
Hancock, W.W., Buelow, R., Sayegh, M.H. & Turka, L.A. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nature Med. 4, 1392–1396 (1998).
Ishikawa, K. et al. Heme oxygenase-1 inhibits atherogenesis in Watanabe heritable hyperlipidemic rabbits. Circulation 104, 1831–1836 (2001).
Juan, S.H. et al. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation 104, 1519–1525 (2001).
Lee, P.J., Alam, J., Wiegand, G.W. & Choi, A.M. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc. Natl. Acad. Sci. USA 93, 10393–81039 (1996).
Pinsky, D.J. et al. Coordinated induction of plasminogen activator inhibitor-1 (PAI-1) and inhibition of plasminogen activator gene expression by hypoxia promotes pulmonary vascular fibrin deposition. J. Clin. Invest. 102, 919–928 (1998).
Fingerle, J., Johnson, R., Clowes, A.W., Majesky, M.W. & Reidy, M.A. Role of platelets in SMC proliferation and migration after vascular injury in rat carotid artery. Proc. Natl. Acad. Sci. USA 86, 8412–8416 (1989).
Lindner, V., Fingerle, J. & Reidy, M.A. Mouse model of arterial injury. Circ. Res. 73, 792–796 (1993).
Reidy, M.A., Fingerle, J. & Lindner, V. Factors controlling the development of arterial lesions after injury. Circulation 86, 43–46 (1992).
Yutani, C. et al. Coronary atherosclerosis and interventions: pathological sequences and restenosis. Pathol. Int. 49, 273–290 (1999).
Lu, H.-T. et al. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 18, 1845–1857 (1999).
Laubach, V.E., Shesely, E.G., Smithies, O. & Sherman, P.A. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc. Natl. Acad. Sci. USA 92, 10688–10689 (1995).
Shesely, E.G. et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 93, 13176–13181 (1996).
Acknowledgements
This work was supported by a grant from the Roche Organ Transplantation Research Foundation (ROTRF; 998521355) awarded to M.P.S.; an Atorvastatin Research Award (AHA) sponsored by Pfizer awarded to M.P.S.; NIH grants HL67040 (M.P.S.), HL58688 (F.H.B.), HL53458 (A.U.), HL60234 (A.M.K.C.), HL5785405 (E.T.); American Heart Association grants 9740246N (A.M.K.C.) and 0160332U (L.E.O.); an AHA sponsored by Pfizer and awarded to L.E.O.; and an Ethicon-Society of University Surgeons Resident Research Award (awarded to B.S.Z.). M.H. was partially supported by a fellowship grant awarded by the Japan Science and Technology Corporation (JST). F.H.B. is the Lewis Thomas Professor at the Harvard Medical School and is a paid consultant for Novartis Pharma.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Otterbein, L., Zuckerbraun, B., Haga, M. et al. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med 9, 183–190 (2003). https://doi.org/10.1038/nm817
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm817