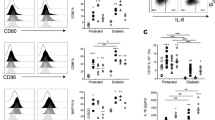
Abstract
Type 1 diabetes develops over many years and is characterized ultimately by the destruction of insulin-producing pancreatic beta cells by autoreactive T cells. Nonetheless, the role of innate cells in the initiation of this disease remains poorly understood. Here, we show that in young female nonobese diabetic mice, physiological beta cell death induces the recruitment and activation of B-1a cells, neutrophils and plasmacytoid dendritic cells (pDCs) to the pancreas. Activated B-1a cells secrete IgGs specific for double-stranded DNA. IgGs activate neutrophils to release DNA-binding cathelicidin-related antimicrobial peptide (CRAMP), which binds self DNA. Then, self DNA, DNA-specific IgG and CRAMP peptide activate pDCs through the Toll-like receptor 9–myeloid differentiation factor 88 pathway, leading to interferon-α production in pancreatic islets. We further demonstrate through the use of depleting treatments that B-1a cells, neutrophils and IFN-α–producing pDCs are required for the initiation of the diabetogenic T cell response and type 1 diabetes development. These findings reveal that an innate immune cell crosstalk takes place in the pancreas of young NOD mice and leads to the initiation of T1D.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Coppieters, K.T. et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J. Exp. Med. 209, 51–60 (2012).
Lehuen, A., Diana, J., Zaccone, P. & Cooke, A. Immune cell crosstalk in type 1 diabetes. Nat. Rev. Immunol. 10, 501–513 (2010).
Reizis, B., Bunin, A., Ghosh, H.S., Lewis, K.L. & Sisirak, V. Plasmacytoid dendritic cells: recent progress and open questions. Annu. Rev. Immunol. 29, 163–183 (2011).
Gilliet, M., Cao, W. & Liu, Y.J. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 8, 594–606 (2008).
Swiecki, M. & Colonna, M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol. Rev. 234, 142–162 (2010).
Fabris, P. et al. Insulin-dependent diabetes mellitus during α-interferon therapy for chronic viral hepatitis. J. Hepatol. 28, 514–517 (1998).
Guerci, A.P. et al. Onset of insulin-dependent diabetes mellitus after interferon-alfa therapy for hairy cell leukaemia. Lancet 343, 1167–1168 (1994).
Allen, J.S. et al. Plasmacytoid dendritic cells are proportionally expanded at diagnosis of type 1 diabetes and enhance islet autoantigen presentation to T-cells through immune complex capture. Diabetes 58, 138–145 (2009).
Winkler, C. et al. An interferon-induced helicase (IFIH1) gene polymorphism associates with different rates of progression from autoimmunity to type 1 diabetes. Diabetes 60, 685–690 (2011).
Stewart, T.A. et al. Induction of type I diabetes by interferon-α in transgenic mice. Science 260, 1942–1946 (1993).
Alba, A. et al. IFN-β accelerates autoimmune type 1 diabetes in nonobese diabetic mice and breaks the tolerance to beta cells in nondiabetes-prone mice. J. Immunol. 173, 6667–6675 (2004).
Trudeau, J.D. et al. Neonatal beta-cell apoptosis: a trigger for autoimmune diabetes? Diabetes 49, 1–7 (2000).
Mathis, D., Vence, L. & Benoist, C. Beta-cell death during progression to diabetes. Nature 414, 792–798 (2001).
Turley, S., Poirot, L., Hattori, M., Benoist, C. & Mathis, D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J. Exp. Med. 198, 1527–1537 (2003).
Bock, T., Kyhnel, A., Pakkenberg, B. & Buschard, K. The postnatal growth of the beta-cell mass in pigs. J. Endocrinol. 179, 245–252 (2003).
Kassem, S.A., Ariel, I., Thornton, P.S., Scheimberg, I. & Glaser, B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 49, 1325–1333 (2000).
O'Brien, B.A. et al. A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J. Autoimmun. 26, 104–115 (2006).
Baumgarth, N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 11, 34–46 (2011).
Kendall, P.L., Woodward, E.J., Hulbert, C. & Thomas, J.W. Peritoneal B cells govern the outcome of diabetes in non-obese diabetic mice. Eur. J. Immunol. 34, 2387–2395 (2004).
Mantovani, A., Cassatella, M.A., Costantini, C. & Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531 (2011).
Lande, R. et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA–peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra19 (2011).
Garcia-Romo, G.S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20 (2011).
Lande, R. & Gilliet, M. Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses. Ann. NY Acad. Sci. 1183, 89–103 (2010).
Fabris, P. et al. Type 1 diabetes mellitus in patients with chronic hepatitis C before and after interferon therapy. Aliment. Pharmacol. Ther. 18, 549–558 (2003).
Li, Q. et al. Interferon-α initiates type 1 diabetes in nonobese diabetic mice. Proc. Natl. Acad. Sci. USA 105, 12439–12444 (2008).
Li, Q. & McDevitt, H.O. The role of interferon α in initiation of type I diabetes in the NOD mouse. Clin. Immunol. 140, 3–7 (2011).
Peng, R.H., Paek, E., Xia, C.Q., Tennyson, N. & Clare-Salzler, M.J. Heightened interferon-α/β response causes myeloid cell dysfunction and promotes T1D pathogenesis in NOD mice. Ann. NY Acad. Sci. 1079, 99–102 (2006).
Sobel, D.O. & Ahvazi, B. α-interferon inhibits the development of diabetes in NOD mice. Diabetes 47, 1867–1872 (1998).
Sobel, D.O. et al. Low dose poly I:C prevents diabetes in the diabetes prone BB rat. J. Autoimmun. 11, 343–352 (1998).
Brod, S.A., Malone, M., Darcan, S., Papolla, M. & Nelson, L. Ingested interferon α suppresses type I diabetes in non-obese diabetic mice. Diabetologia 41, 1227–1232 (1998).
Tanaka-Kataoka, M. et al. Oral use of interferon-α delays the onset of insulin-dependent diabetes mellitus in nonobese diabetes mice. J. Interferon Cytokine Res. 19, 877–879 (1999).
Serreze, D.V., Hamaguchi, K. & Leiter, E.H. Immunostimulation circumvents diabetes in NOD/Lt mice. J. Autoimmun. 2, 759–776 (1989).
Zhou, R., Wei, H. & Tian, Z. NK3-like NK cells are involved in protective effect of polyinosinic-polycytidylic acid on type 1 diabetes in nonobese diabetic mice. J. Immunol. 178, 2141–2147 (2007).
Ewel, C.H., Sobel, D.O., Zeligs, B.J. & Bellanti, J.A. Poly I:C accelerates development of diabetes mellitus in diabetes-prone BB rat. Diabetes 41, 1016–1021 (1992).
Sobel, D.O. et al. Poly I:C induces development of diabetes mellitus in BB rat. Diabetes 41, 515–520 (1992).
Huang, X., Hultgren, B., Dybdal, N. & Stewart, T.A. Islet expression of interferon-α precedes diabetes in both the BB rat and streptozotocin-treated mice. Immunity 1, 469–478 (1994).
Theofilopoulos, A.N., Kono, D.H., Beutler, B. & Baccala, R. Intracellular nucleic acid sensors and autoimmunity. J. Interferon Cytokine Res. 31, 867–886 (2011).
Desmet, C.J. & Ishii, K.J. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat. Rev. Immunol. 12, 479–491 (2012).
Lang, K.S. et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat. Med. 11, 138–145 (2005).
Aune, T.M. & Pierce, C.W. Activation of a suppressor T-cell pathway by interferon. Proc. Natl. Acad. Sci. USA 79, 3808–3812 (1982).
Mujtaba, M.G., Soos, J.M. & Johnson, H.M. CD4 T suppressor cells mediate interferon τ protection against experimental allergic encephalomyelitis. J. Neuroimmunol. 75, 35–42 (1997).
Teige, I., Liu, Y. & Issazadeh-Navikas, S. IFN-β inhibits T cell activation capacity of central nervous system APCs. J. Immunol. 177, 3542–3553 (2006).
González-Navajas, J.M., Lee, J., David, M. & Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 12, 125–135 (2012).
Kared, H. et al. Treatment with granulocyte colony-stimulating factor prevents diabetes in NOD mice by recruiting plasmacytoid dendritic cells and functional CD4+CD25+ regulatory T-cells. Diabetes 54, 78–84 (2005).
Saxena, V., Ondr, J.K., Magnusen, A.F., Munn, D.H. & Katz, J.D. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J. Immunol. 179, 5041–5053 (2007).
Diana, J. et al. Viral infection prevents diabetes by inducing regulatory T cells through NKT cell–plasmacytoid dendritic cell interplay. J. Exp. Med. 208, 729–745 (2011).
Diana, J. et al. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity 30, 289–299 (2009).
Mallone, R. & Brezar, V. To B or not to B: (anti)bodies of evidence on the crime scene of type 1 diabetes? Diabetes 60, 2020–2022 (2011).
De Filippo, G. et al. Increased CD5+CD19+ B lymphocytes at the onset of type 1 diabetes in children. Acta Diabetol. 34, 271–274 (1997).
Habib, T. et al. Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant. J. Immunol. 188, 487–496 (2012).
Shirai, T., Okada, T. & Hirose, S. Genetic regulation of CD5+ B cells in autoimmune disease and in chronic lymphocytic leukemia. Ann. NY Acad. Sci. 651, 509–526 (1992).
Burastero, S.E., Casali, P., Wilder, R.L. & Notkins, A.L. Monoreactive high affinity and polyreactive low affinity rheumatoid factors are produced by CD5+ B cells from patients with rheumatoid arthritis. J. Exp. Med. 168, 1979–1992 (1988).
Thomas, J.W., Kendall, P.L. & Mitchell, H.G. The natural autoantibody repertoire of nonobese diabetic mice is highly active. J. Immunol. 169, 6617–6624 (2002).
Kessenbrock, K. et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 15, 623–625 (2009).
Guiducci, C. et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J. Exp. Med. 207, 2931–2942 (2010).
Kono, H. & Rock, K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 8, 279–289 (2008).
Hanayama, R. et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8–deficient mice. Science 304, 1147–1150 (2004).
Todd, J.A. Etiology of type 1 diabetes. Immunity 32, 457–467 (2010).
Aumeunier, A. et al. Systemic Toll-like receptor stimulation suppresses experimental allergic asthma and autoimmune diabetes in NOD mice. PLoS ONE 5, e11484 (2010).
Murakami, M., Yoshioka, H., Shirai, T., Tsubata, T. & Honjo, T. Prevention of autoimmune symptoms in autoimmune-prone mice by elimination of B-1 cells. Int. Immunol. 7, 877–882 (1995).
Acknowledgements
We thank N. Charles (INSERM UMR-S 699, Paris Diderot University) for dsDNA-specific immunoglobulin ELISA, M. Colonna (Department of Pathology and Immunology, Washington University School of Medicine) for m927 mAb, S. Mecheri (Biology of Host Parasite Interactions Unit, Pasteur Institute) for NIMP-14 mAb, S. Muller (CNRS, Institut de Biologie Moléculaire et Cellulaire, UPR9021) for serum from NZB/W F1 mice, J. Ravetch (Laboratory of Molecular Genetics and Immunology, Rockefeller University) for FcγRIV-specific mAb, N. Thieblemont (CNRS UMR 8147, Paris Descartes University) for Myd88−/− NOD mice and all for their expertise. We thank F. Boutillon for technical assistance and the staff of the INSERM U986 mouse facility for help in animal care. Y.S. is supported by a doctoral fellowship from the Region Ile de France. This work was supported by funds from INSERM, Centre National de la Recherche Scientifique, ANR-05-PCOD009-01, ANR-09-GENO-023 and Labex INFLAMEX to A.L. A.L. is recipient of an APHP-CNRS Contrat Hospitalier de Recherche Translationelle.
Author information
Authors and Affiliations
Contributions
J.D. initiated and led the whole project, coordinated with different investigators, designed and performed experiments, analyzed the data and wrote the manuscript; Y.S. designed and performed experiments, analyzed the data and wrote the manuscript; L.F. did confocal microscopy experiments, analyzed the data and wrote the manuscript; L.B. provided technical assistance; B.A. and F.B. provided intellectual input and key reagents for neutrophil and pDC analysis; and A.L. was responsible for project planning, data analysis, discussion and writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–18 (PDF 4060 kb)
Rights and permissions
About this article
Cite this article
Diana, J., Simoni, Y., Furio, L. et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med 19, 65–73 (2013). https://doi.org/10.1038/nm.3042
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3042
This article is cited by
-
Cyclical palmitoylation regulates TLR9 signalling and systemic autoimmunity in mice
Nature Communications (2024)
-
Exploration of common genomic signatures of systemic juvenile rheumatoid arthritis and type 1 diabetes
Scientific Reports (2023)
-
B1-cell-produced anti-phosphatidylserine antibodies contribute to lupus nephritis development via TLR-mediated Syk activation
Cellular & Molecular Immunology (2023)
-
Gene expression profiling in NOD mice reveals that B cells are highly educated by the pancreatic environment during autoimmune diabetes
Diabetologia (2023)
-
Changes of B cell subsets in different types of diabetes and its effect on the progression of latent autoimmune diabetes in adults
Endocrine (2023)