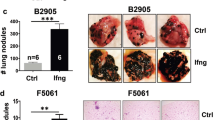
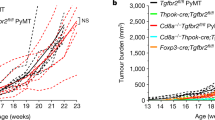
Abstract
'Cancer immunoediting' is a process wherein the immune system protects hosts against tumor development and facilitates outgrowth of tumors with reduced immunogenicity. Although interferon-γ (IFN-γ) is known to be involved in this process, the involvement of type I interferons (IFN-α/β) has not been elucidated. We now show that, like IFN-γ, endogenously produced IFN-α/β was required for the prevention of the growth of primary carcinogen–induced and transplantable tumors. Although tumor cells are important IFN-γ targets, they are not functionally relevant sites of the actions of the type I interferons. Instead, host hematopoietic cells are critical IFN-α/β targets during development of protective antitumor responses. Therefore, type I interferons are important components of the cancer immunoediting process and function in a way that does not completely overlap the functions of IFN-γ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Change history
24 June 2005
Changed label on Fig. 4a and 4c
Notes
NOTE: In the version of this article initially published online, the graphs in Figure 4a,c were labeled incorrectly. The graphs are labeled correctly here. The error has been corrected in the HTML version of the article. This correction has been appended to the PDF and print versions.
References
Shankaran, V. et al. IFN-γ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111 (2001).
Dunn, G.P. et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3, 991–998 (2002).
Dunn, G.P., Old, L.J. & Schreiber, R.D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22, 329–360 (2004).
Dunn, G.P., Old, L.J. & Schreiber, R.D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21, 137–148 (2004).
Dighe, A.S., Richards, E., Old, L.J. & Schreiber, R.D. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN-γ receptors. Immunity 1, 447–456 (1994).
Kaplan, D.H. et al. Demonstration of an interferon-γ-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. USA 95, 7556–7561 (1998).
Ikeda, H., Old, L.J. & Schreiber, R.D. The roles of IFN-γ in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 13, 95–109 (2002).
Mumberg, D. et al. CD4+ T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-γ. Proc. Natl. Acad. Sci. USA 96, 8633–8638 (1999).
Fallarino, F. & Gajewski, T.F. Cutting edge: differentiation of antitumor CTL in vivo requires host expression of Stat1. J. Immunol. 163, 4109–4113 (1999).
Gresser, I. et al. Injection of mice with antibody to interferon enhances the growth of transplantable murine tumors. J. Exp. Med. 158, 2095–2107 (1983).
Gresser, I., Bandu, M.T. & Brouty-Boye, D. Interferon and cell division. IX. Interferon-resistant L1210 cells: characteristics and origin. J. Natl. Cancer Inst. 52, 553–559 (1974).
Affabris, E. et al. Molecular mechanisms of action of interferons in the Friend virus-induced leukemia cell system. Haematologica 72, 76–78 (1987).
Gresser, I. et al. Antibody to mouse interferon α/β abrogates resistance to the multiplication of Friend erythroleukemia cells in the livers of allogeneic mice. J. Exp. Med. 168, 1271–1291 (1988).
Belardelli, F., Ferrantini, M., Proietti, E. & Kirkwood, J.M. Interferon-α in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 13, 119–134 (2002).
Qin, Z., Kim, H.J., Hemme, J. & Blankenstein, T. Inhibition of methylcholanthrene-induced carcinogenesis by an interferon-γ receptor-dependent foreign body reaction. J. Exp. Med. 195, 1479–1490 (2002).
Dighe, A.S., Farrar, M.A. & Schreiber, R.D. Inhibition of cellular responsiveness to interferon-γ (IFN-γ) induced by overexpression of inactive forms of the IFN-γ receptor. J. Biol. Chem. 268, 10645–10653 (1993).
Qin, Z. & Blankenstein, T. CD4+ T Cell-mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN-γ receptor expression by nonhematopoietic cells. Immunity 12, 677–686 (2000).
Le Bon, A. & Tough, D.F. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14, 432–436 (2002).
Gallucci, S., Lolkema, M. & Matzinger, P. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 5, 1249–1255 (1999).
Montoya, M. et al. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 99, 3263–3271 (2002).
Le Bon, A. et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4, 1009–1015 (2003).
Le Bon, A. et al. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14, 461–470 (2001).
Wu, J. & Lanier, L.L. Natural killer cells and cancer. Adv. Cancer Res. 90, 127–156 (2003).
Sato, K. et al. Antiviral response by natural killer cells through TRAIL gene induction by IFN-α/β. Eur. J. Immunol. 31, 3138–3146 (2001).
Marrack, P., Kappler, J. & Mitchell, T. Type I interferons keep activated T cells alive. J. Exp. Med. 189, 521–530 (1999).
Zhang, X. et al. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8, 591–599 (1998).
Takaoka, A. et al. Integration of interferon-α/β signalling to p53 responses in tumour suppression and antiviral defence. Nature 424, 516–523 (2003).
Levine, A.J. p53, the cellular gatekeeper for growth and division. Cell 88, 323–331 (1997).
Tough, D.F. Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leuk. Lymphoma 45, 257–264 (2004).
Colonna, M., Krug, A. & Cella, M. Interferon-producing cells: on the front line in immune responses against pathogens. Curr. Opin. Immunol. 14, 373–379 (2002).
Colonna, M., Trinchieri, G. & Liu, Y.J. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5, 1219–1226 (2004).
Taniguchi, T. & Takaoka, A. A weak signal for strong responses: interferon-α/β revisited. Nat. Rev. Mol. Cell Biol. 2, 378–386 (2001).
Soos, J.M. & Szente, B.E. Type I interferons. in The Cytokine Handbook Vol. 1 (eds. Lotz, M.T. & Thomson, A.W.) 549–566 (Academic Press, 2003).
Enzler, T. et al. Deficiencies of GM-CSF and interferon γ link inflammation and cancer. J. Exp. Med. 197, 1213–1219 (2003).
Lesinski, G.B. et al. The antitumor effects of IFN-α are abrogated in a STAT1-deficient mouse. J. Clin. Invest. 112, 170–180 (2003).
Belardelli, F., Gresser, I., Maury, C. & Maunoury, M.T. Antitumor effects of interferon in mice injected with interferon-sensitive and interferon-resistant Friend leukemia cells. I. Int. J. Cancer 30, 813–820 (1982).
Afkarian, M. et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat. Immunol. 3, 549–557 (2002).
Liu, F., Song, Y. & Liu, D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 6, 1258–1266 (1999).
Zhang, G., Budker, V. & Wolff, J.A. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum. Gene Ther. 10, 1735–1737 (1999).
Sheehan, K.C. et al. Monoclonal antibodies specific for murine p55 and p75 tumor necrosis factor receptors: identification of a novel in vivo role for p75. J. Exp. Med. 181, 607–617 (1995).
Kondo, M. et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu. Rev. Immunol. 21, 759–806 (2003).
Pear, W.S. et al. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood 92, 3780–3792 (1998).
Acknowledgements
We thank L. Old of the Ludwig Institute for Cancer Research; members of the Schreiber laboratory for discussions throughout this work; W. Yokoyama and M. Orihuela and the Speed Congenics Core facility of the Rheumatic Diseases Core Center at Washington University for genetic marker analyses; and D. Kreamalmeyer, K. Kooi and T. Irwin for assistance with animal work. Supported by the National Cancer Institute (CA43059 and CA107527), Ludwig Institute for Cancer Research and Cancer Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Selective reconsitution of IFNγ or IFNα/β responsiveness in the GAR4 tumor. (PDF 53 kb)
Supplementary Fig. 2
Microsatellite analysis indicates that IFNAR−/− mice are on the 129/SvPAS (129S2) genetic background. (PDF 127 kb)
Rights and permissions
About this article
Cite this article
Dunn, G., Bruce, A., Sheehan, K. et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol 6, 722–729 (2005). https://doi.org/10.1038/ni1213
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1213
This article is cited by
-
Targeting cGAS/STING signaling-mediated myeloid immune cell dysfunction in TIME
Journal of Biomedical Science (2023)
-
Immunometabolism in biofilm infection: lessons from cancer
Molecular Medicine (2022)
-
Emerging strategies in targeting tumor-resident myeloid cells for cancer immunotherapy
Journal of Hematology & Oncology (2022)
-
The stimulator of interferon genes (STING) agonists for treating acute myeloid leukemia (AML): current knowledge and future outlook
Clinical and Translational Oncology (2022)
-
New Immuno-oncology Targets and Resistance Mechanisms
Current Treatment Options in Oncology (2022)