Abstract
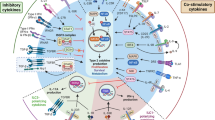
Group 2 innate lymphoid cells (ILC2s) secrete type 2 cytokines, which protect against parasites but can also contribute to a variety of inflammatory airway diseases. We report here that interleukin 1β (IL-1β) directly activated human ILC2s and that IL-12 induced the conversion of these activated ILC2s into interferon-γ (IFN-γ)-producing ILC1s, which was reversed by IL-4. The plasticity of ILCs was manifested in diseased tissues of patients with severe chronic obstructive pulmonary disease (COPD) or chronic rhinosinusitis with nasal polyps (CRSwNP), which displayed IL-12 or IL-4 signatures and the accumulation of ILC1s or ILC2s, respectively. Eosinophils were a major cellular source of IL-4, which revealed cross-talk between IL-5-producing ILC2s and IL-4-producing eosinophils. We propose that IL-12 and IL-4 govern ILC2 functional identity and that their imbalance results in the perpetuation of type 1 or type 2 inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others

References
Barnes, P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 8, 183–192 (2008).
Hulse, K.E., Stevens, W.W., Tan, B.K. & Schleimer, R.P. Pathogenesis of nasal polyposis. Clin. Exp. Allergy 45, 328–346 (2015).
Nagarkar, D.R. et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J. Allergy. Clin. Immunol. 132, 593–600 (2013).
Reh, D.D., Wang, Y., Ramanathan, M. Jr. & Lane, A.P. Treatment-recalcitrant chronic rhinosinusitis with polyps is associated with altered epithelial cell expression of interleukin-33. Am. J. Rhinol. Allergy 24, 105–109 (2010).
Barnes, P.J. The cytokine network in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 41, 631–638 (2009).
Kearley, J. et al. Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin-33-dependent response to infection. Immunity 42, 566–579 (2015).
Holtzman, M.J., Byers, D.E., Alexander-Brett, J. & Wang, X. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat. Rev. Immunol. 14, 686–698 (2014).
McKenzie, A.N., Spits, H. & Eberl, G. Innate lymphoid cells in inflammation and immunity. Immunity 41, 366–374 (2014).
Artis, D. & Spits, H. The biology of innate lymphoid cells. Nature 517, 293–301 (2015).
Buonocore, S. et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375 (2010).
Klose, C.S. et al. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature 494, 261–265 (2013).
Bernink, J.H. et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 14, 221–229 (2013).
Bartemes, K.R., Kephart, G.M., Fox, S.J. & Kita, H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol 134, 671–678 (2014).
Nagakumar, P. et al. Type 2 innate lymphoid cells in induced sputum from children with severe asthma. J. Allergy Clin. Immunol. 137, 624–626.e6 (2015).
Mjösberg, J.M. et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 12, 1055–1062 (2011).
Salimi, M. et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 210, 2939–2950 (2013).
Teunissen, M.B. et al. Composition of innate lymphoid cell subsets in the human skin: enrichment of NCR+ ILC3 in lesional skin and blood of psoriasis patients. J. Invest. Dermatol. 134, 2351–2360 (2014).
Kim, H.Y. et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat. Med. 20, 54–61 (2014).
Gasteiger, G., Fan, X., Dikiy, S., Lee, S.Y. & Rudensky, A.Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350, 981–985 (2015).
Bernink, J.H. et al. Interleukin-12 and -23 control plasticity of CD127+ group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 43, 146–160 (2015).
Vonarbourg, C. et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt+ innate lymphocytes. Immunity 33, 736–751 (2010).
Hazenberg, M.D. & Spits, H. Human innate lymphoid cells. Blood 124, 700–709 (2014).
Roediger, B. & Weninger, W. Group 2 innate lymphoid cells in the regulation of immune responses. Adv. Immunol. 125, 111–154 (2015).
Brestoff, J.R. et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246 (2015).
Mjösberg, J. et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity 37, 649–659 (2012).
Gimeno, R. et al. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2−/− γc−/− mice: functional inactivation of p53 in developing T cells. Blood 104, 3886–3893 (2004).
Hughes, T. et al. Interleukin-1β selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity 32, 803–814 (2010).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Hackett, T.L., Shaheen, F., Zhou, S., Wright, J.L. & Churg, A. Fibroblast signal transducer and activator of transcription 4 drives cigarette smoke-induced airway fibrosis. Am. J. Respir. Cell Mol. Biol. 51, 830–839 (2014).
Neurath, M.F., Finotto, S. & Glimcher, L.H. The role of Th1/Th2 polarization in mucosal immunity. Nat. Med. 8, 567–573 (2002).
Miljkovic, D. et al. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy 69, 1154–1161 (2014).
Voehringer, D., Shinkai, K. & Locksley, R.M. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity 20, 267–277 (2004).
Sojka, D.K. et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife 3, e01659 (2014).
Silver, J.S. et al. Inflammatory triggers associated with COPD exacerbations orchestrate ILC2 plasticity in the lung. Nat. Immunol. doi:10.1038/ni.3443 (2016).10.1038/ni.3443
Barnes, P.J. Therapeutic approaches to asthma-chronic obstructive pulmonary disease overlap syndromes. J. Allergy Clin. Immunol. 136, 531–545 (2015).
Molofsky, A.B. et al. Interleukin-33 and interferon-γ counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity 43, 161–174 (2015).
Nussbaum, J.C. et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502, 245–248 (2013).
Southworth, T. et al. IFN-γ synergistically enhances LPS signalling in alveolar macrophages from COPD patients and controls by corticosteroid-resistant STAT1 activation. Br. J. Pharmacol. 166, 2070–2083 (2012).
Lee, B.J. et al. Protective effects of basic fibroblast growth factor in the development of emphysema induced by interferon-γ. Exp. Mol. Med. 43, 169–178 (2011).
Bachert, C. et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. J. Am. Med. Assoc. 315, 469–479 (2016).
Wenzel, S. et al. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 368, 2455–2466 (2013).
Beck, L.A. et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 371, 130–139 (2014).
Gevaert, P. et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J. Allergy Clin. Immunol. 118, 1133–1141 (2006).
Gevaert, P. et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J. Allergy Clin. Immunol. 128, 989–995 (2011).
Corren, J. et al. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 365, 1088–1098 (2011).
Abt, M.C. et al. innate immune defenses mediated by two ILC subsets are critical for protection against acute Clostridium difficile infection. Cell Host Microbe 18, 27–37 (2015).
Acknowledgements
We thank B. Hooibrink for help with flow cytometry; staff of the Bloemenhove clinic in Heemstede, the Netherlands, for fetal tissues; K. Weijer for processing fetal material; B. Dierdorp and T. Dekker for performing the multiplex assay; E. van Rijnstra for help with the animal experiments; E. de Groot, D. van Egmond and Britt-Marie Nilsson for immunohistochemical staining; C. Loftus (Medetect AB) for computer simulations of spatial cell distributions. Y. Pineros for isolating eosinophils, and J. Fergusson for critical reading of the manuscript. Supported by the European Research Council (Advanced ERC grant 341038-AsthmaVir).
Author information
Authors and Affiliations
Contributions
S.M.B. and J.H.B. designed the study, did experiments, analyzed the data and wrote the manuscript; M.N., J.G. and M.M.S. did experiments and analyzed the data; K.G., M.B., J.V. and J.M.M. did experiments; C.M.v.D. and W.F. provided nasal tissue; R.L. provided BAL fluid cells and analyzed data; R.E.J. and P.H. provided lung tissue; J.S.E. performed histological assessments; H.S. designed the study, analyzed data and wrote the manuscript ; X.R.R. designed the study, did experiments, analyzed the data and wrote the manuscript; and all authors critically read the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Gating strategy for the detection of ILCs in human lungs and nasal polyps, and the expression of cell-surface proteins on nasal-polyp ILCs.
(a) Flow cytometry analysis of human lung and nasal polyp tissue of CD45, Lin (CD1a, CD3, CD14, CD16, CD19, CD34, CD123, BDCA2, FcɛR1α, TCRαβ, TCRγδ), CD127, CD161, c-Kit, CRTH2, and NKp44 to detect ILC1 (CRTH2- c-Kit- NKp44-), ILC2 (CRTH2+ c-Kit+/-), and ILC3 (CRTH2- c-Kit+ NKp44+/-). (b) Flow cytometry analyzing the expression of CRTH2, IL7Rα, CD161, ST2, TSLPR, IL17RB, CD25, KLRG1, IL4R, IL9R and ICOS in ILC1 (black), ILC2 (red) and ILC3 (grey line) from nasal polyp tissue. Isotype-matched control antibody is shown as grey shaded curve. Data are representative of at least three different donors (a,b).
Supplementary Figure 2 Repopulation of Il2rg–/– NOD-SCID mice with human hematopoietic stem cells and purity of expanded ILC2 populations.
(a) Flow cytometry analysis of human and murine CD45 expression in blood, lung and spleen of Il2rg–/– NOD-SCID mice eight weeks after engraftment with human hematopoietic stem cells. (b) c-Kit and CRTH2 expression after resorting of ILC2 that were expanded for four weeks with irradiated allogeneic peripheral blood mononuclear cells, irradiated Epstein-Barr virus–transformed JY human B cells, phytohemagglutinin and IL-2. (c) CRTH2 expression after culturing CTV labeled ILC2 for 4 days with IL-2. Data are representative of six mice (a) or five different experiments (b).
Supplementary Figure 3 Reactivation of ILC2 cells with IL-33 and TSLP and transdifferentiation of lung ILC2 cells.
(a) Flow cytometry analysis of c-Kit and CRTH2 expression on blood ILC2 (left panel) and IL-5 and IL-13 production (right panel) upon stimulation for five days with IL-2, IL-2 + IL-33 + TSLP. Cells were washed and stimulated for another four days with IL-2 + IL-33 + TSLP. (b) Expression of IL12RB2 on ILC1, ILC2, ILC3 and NK cells as measured by qPCR. (c) IL-5 and IL-13 production by blood ILC2, ILC1, and ILC2 expanded with IL-2, IL-1β and IL-12 after culture for 4 days with IL-2, IL-33, and TSLP, (d) intracellular IL-5 and IFN-γ production by cells as under cafter culture for 4 days with IL-2, IL-12, and IL-18. (e) Flow cytometry analysis of c-Kit and CRTH2 expression on lung ILC2 directly after isolation and upon stimulation with IL-2, IL-33, and IL-12 for seven days and IFN-γ production of lung c-Kit- ex-ILC2 obtained upon stimulation with IL-2, IL-33, and IL-12 for seven days. (f) Isolated ILC2 from lung cultured for 6 days either with IL-2, or IL-2, IL-33, and TSLP, or with IL-2, IL-33, TSLP, and IL-12. Cells were analyzed for c-Kit and CRTH2 expression (left panels), percentage of c-Kit- ex-ILC2 generated in above mentioned activation conditions (right panel). Data are representative of three (a,c,d,e,f) or four (b) different donors.
Supplementary Figure 4 Stimulation of ILC1 and ILC3 cells with IL-4, and gating strategy for BAL-fluid ILCs.
(a) Flow cytometry analysis of c-Kit and CRTH2 expression on blood ILC1 and ILC3 upon stimulation for five days with IL-2 or IL-2 and IL-4. (b) Gating strategy for ILC detection and ILC distribution in bronchoalveolar lavage (BAL) fluid. Data are representative of three individual experiments (a) or five different samples (b).
Supplementary Figure 5 Gating strategy for basophils, mast cells and eosinophils in nasal-polyp and turbinate tissue.
Polyp and turbinate tissue was analyzed by flow cytometry for CD45, CD3, CD123, FcɛRI, c-Kit, CD203c and Siglec8. Basophils were gated as CD3- CD123+ FcɛRI+ CD203c+, mast cells as c-Kit+ FcɛRI+ CD203c+, and eosinophils as SSChigh Siglec8+. Data are representative of five polyp and two turbinate samples.
Supplementary Figure 6 ILC2 spatial distribution.
Using the known x,y coordinates for each eosinophil, the true observed accumulated eosinophil counts within a fixed close space surrounding each ILC2 cell were calculated and compared to computer simulations of random ILC distributions. The true accumulated eosinophils count within a 300 µm radius around each of the 20 ILC in Figure 8b (n=668 ILC-associated eosinophils) was compared to the combined eosinophil counts calculated for simulated 250 000 cases of computer-generated random distributions of the same number of fictive ILCs (mean combined eosinophil count = 139, range 35-452).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Tables 1–5 (PDF 1076 kb)
Rights and permissions
About this article
Cite this article
Bal, S., Bernink, J., Nagasawa, M. et al. IL-1β, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol 17, 636–645 (2016). https://doi.org/10.1038/ni.3444
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3444
This article is cited by
-
A diversity of novel type-2 innate lymphoid cell subpopulations revealed during tumour expansion
Communications Biology (2024)
-
Group 2 innate lymphoid cells and their surrounding environment
Inflammation and Regeneration (2023)
-
Pathogenesis of allergic diseases and implications for therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
Versatile roles of innate lymphoid cells at the mucosal barrier: from homeostasis to pathological inflammation
Experimental & Molecular Medicine (2023)
-
The modulation of pulmonary group 2 innate lymphoid cell function in asthma: from inflammatory mediators to environmental and metabolic factors
Experimental & Molecular Medicine (2023)