Abstract
The asymmetric partitioning of fate-determining proteins has been shown to contribute to the generation of CD8+ effector and memory T cell precursors. Here we demonstrate the asymmetric partitioning of mTORC1 activity after the activation of naive CD8+ T cells. This results in the generation of two daughter T cells, one of which shows increased mTORC1 activity, increased glycolytic activity and increased expression of effector molecules. The other daughter T cell has relatively low mTORC1 activity and increased lipid metabolism, expresses increased amounts of anti-apoptotic molecules and subsequently displays enhanced long-term survival. Mechanistically, we demonstrate a link between T cell antigen receptor (TCR)-induced asymmetric expression of amino acid transporters and RagC-mediated translocation of mTOR to the lysosomes. Overall, our data provide important insight into how mTORC1-mediated metabolic reprogramming affects the fate decisions of T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Betschinger, J. & Knoblich, J.A. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 14, R674–R685 (2004).
Chang, J.T. et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 315, 1687–1691 (2007).
Chang, J.T. et al. Asymmetric proteasome segregation as a mechanism for unequal partitioning of the transcription factor T-bet during T lymphocyte division. Immunity 34, 492–504 (2011).
Arsenio, J. et al. Early specification of CD8+ T lymphocyte fates during adaptive immunity revealed by single-cell gene-expression analyses. Nat. Immunol. 15, 365–372 (2014).
Oliaro, J. et al. Asymmetric cell division of T cells upon antigen presentation uses multiple conserved mechanisms. J. Immunol. 185, 367–375 (2010).
Lin, W.H. et al. Asymmetric PI3K signaling driving developmental and regenerative cell fate bifurcation. Cell Rep. 13, 2203–2218 (2015).
Delgoffe, G.M. et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30, 832–844 (2009).
Delgoffe, G.M. et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12, 295–303 (2011).
Lee, K. et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 32, 743–753 (2010).
Heikamp, E.B. et al. The AGC kinase SGK1 regulates TH1 and TH2 differentiation downstream of the mTORC2 complex. Nat. Immunol. 15, 457–464 (2014).
Yang, K. et al. T cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity 39, 1043–1056 (2013).
Pollizzi, K.N., Waickman, A.T., Patel, C.H., Sun, I.H. & Powell, J.D. Cellular size as a means of tracking mTOR activity and cell fate of CD4+ T cells upon antigen recognition. PLoS One 10, e0121710 (2015).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009).
Pearce, E.L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
Rao, R.R., Li, Q., Odunsi, K. & Shrikant, P.A. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity 32, 67–78 (2010).
Pollizzi, K.N. et al. mTORC1 and mTORC2 selectively regulate CD8+ T cell differentiation. J. Clin. Invest. 125, 2090–2108 (2015).
Finlay, D.K. et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J. Exp. Med. 209, 2441–2453 (2012).
MacIver, N.J., Michalek, R.D. & Rathmell, J.C. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 31, 259–283 (2013).
Pollizzi, K.N. & Powell, J.D. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat. Rev. Immunol. 14, 435–446 (2014).
Pollizzi, K.N. & Powell, J.D. Regulation of T cells by mTOR: the known knowns and the known unknowns. Trends Immunol. 36, 13–20 (2015).
Metz, P.J. et al. Regulation of asymmetric division by atypical protein kinase C influences early specification of CD8+ T lymphocyte fates. Sci. Rep. 6, 19182 (2016).
Intlekofer, A.M. et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6, 1236–1244 (2005).
King, C.G. et al. T cell affinity regulates asymmetric division, effector cell differentiation, and tissue pathology. Immunity 37, 709–720 (2012).
van der Windt, G.J. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
van der Windt, G.J. et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc. Natl. Acad. Sci. USA 110, 14336–14341 (2013).
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011).
Gera, J.F. et al. AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J. Biol. Chem. 279, 2737–2746 (2004).
West, M.J., Stoneley, M. & Willis, A.E. Translational induction of the c-myc oncogene via activation of the FRAP/TOR signalling pathway. Oncogene 17, 769–780 (1998).
Sancak, Y. et al. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141, 290–303 (2010).
Zoncu, R. et al. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 334, 678–683 (2011).
Bar-Peled, L., Schweitzer, L.D., Zoncu, R. & Sabatini, D.M. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150, 1196–1208 (2012).
Sinclair, L.V. et al. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 14, 500–508 (2013).
Torrents, D. et al. Identification and characterization of a membrane protein (y+L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y+L. A candidate gene for lysinuric protein intolerance. J. Biol. Chem. 273, 32437–32445 (1998).
Liu, X., Charrier, L., Gewirtz, A., Sitaraman, S. & Merlin, D. CD98 and intracellular adhesion molecule I regulate the activity of amino acid transporter LAT-2 in polarized intestinal epithelia. J. Biol. Chem. 278, 23672–23677 (2003).
Chang, C.H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013).
Sukumar, M. et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Invest. 123, 4479–4488 (2013).
Bannard, O., Kraman, M. & Fearon, D.T. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science 323, 505–509 (2009).
Wherry, E.J. et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4, 225–234 (2003).
Kaech, S.M. & Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 (2012).
Acknowledgements
We thank members of the Powell lab and C. Gamper for critical discussion of the manuscript; B. Smith and T. Stephens for their technical support in microscopy studies; M. Gambello (Emory University, Atlanta, Georgia, USA) for Tsc2loxP mice; and P. Worley (Johns Hopkins University, Baltimore, Maryland, USA) for RhebloxP mice. This work was supported by the NIH (grants AI072677, AI77610 and AI091481 to J.D.P., and grants S10 OD016374 and S10 RR024550 to the JHUSOM Microscope Facility).
Author information
Authors and Affiliations
Contributions
K.N.P., I.-H.S., C.H.P. and J.D.P. planned the experiments, analyzed the data and wrote the manuscript; K.N.P., I.-H.S., C.H.P., Y.-C.L., M.-H.O., A.T.W. and J.W. performed the experiments; G.M.D. and A.T.W. contributed to the experimental design, execution and data interpretation; A.J.T. and R.L.B. provided support for flow cytometry experiments; G.M.D. and J.D.P. conceived the idea for the study; K.N.P., I.-H.S., C.H.P., Y.-C.L., M.-H.O., G.M.D. and J.D.P. provided critical review of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Polarized TCR activation induces asymmetric division of CD8+ T cells with phenotypical and functional differences.
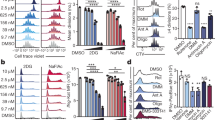
(a-c) As in Fig. 1A, eFluor 450-labeled OT-I cells were adoptively transferred (i.v.) into WT mice and infected with LM-OVA (i.v.). Flow cytometry plots showing CD8 expression (a) and p-S6, CD98, T-bet, and Myc (b) between CD8hi and CD8lo T cells in 1st division and undivided cells. (c) Flow cytometry analysis of Eomes and CD44 expression shown from first division CD8hi (grey) and CD8lo (red) T cells. Naïve cells are shown in black dotted line. (d) Immunoblot analysis of p-4E-BP1 and p-ERK between sorted CD8hi and CD8lo T cells. (e) Flow cytometry of activation markers in CD8hi (grey) and CD8lo (red) T cells from first division after in vitro activation. (f) Confocal images of dividing CD8+ T cells in contact with bone marrow derived APC. Proximal cell in contact is shown on top of the APC. (g) qRT-PCR analysis of mRNA relative expression of Ifng and Prf1 in sorted CD8hi and CD8lo obtained from in vitro activation, n=5 (Ifng), 6 (Prf1). (h) Supernatant was collected from CD8hi and CD8lo T cells 5 h post-sorting. Amount of IFN-γ was measured from the supernatant by ELISA, n=6 (IFN-γ). *P < 0.05; ***P < 0.0005; ****P < 0.0001 (Mann-Whitney t test (g,h). Data are summary plots from at least 2 independent experiments (a-f) or compilation of 2 independent experiments (g,h). Scale bars, 10µm.
Supplementary Figure 2 Polarized T cell activation initiates asymmetric division of CD8hi and CD8lo daughter T cells.
Confocal images of CD8+ T cells activated for 36 h with polarized plate-bound stimuli (top) or activated for 5 h by PMA + ionomycin and rested for additional 31 h (bottom). T Cells were stained with anti-CD8 and anti-p-S6. Statistical analysis was performed comparing p-S6 MFI between CD8hi and CD8lo T cells, n=18 (Platebound) and 19 (PMA + Ionomycin). *P < 0.05; NS, not significant (Wilcoxon rank test). Data are representative of at least 2 independent experiments. Scale bars, 10µm.
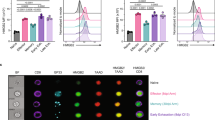
Supplementary Figure 3 CD98 accumulates at the immunological synapse.
OT-I CD8+ T cells were activated in the presence of OVA-I peptide-pulsed bone marrow derived APCs for 19 h. Confocal image of APCs and CD8+ T cells stained with anti-CD98 and anti-talin. Results are representative of 2 independent experiments. Scale bar, 10µm.
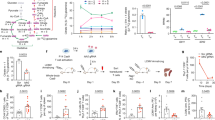
Supplementary Figure 4 mTORC1 activity does not control differential expression of amino acid transporters in an in vivo infection model.
Flow cytometry of LFA-1, CD98, and p-S6 expression gated on adoptive transferred (i.v.) WT or T-Rheb–/– OT-I CD90.1+ CD8+ T cells from LM-OVA-infected WT hosts from first division indicated by proliferation dye. Data are representative of 2 independent experiments.
Supplementary Figure 5 Model of asymmetric division.
CD8hi proximal T cells have increased levels of amino acid transporter SLC7A5, resulting in an increased influx of amino acids that correlates with increased mTOR activity. This supports robust effector function in CD8hi daughter cells. In contrast, CD8lo distal cells have lower levels of mTOR activity and are destined to become memory cells.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 2229 kb)
Rights and permissions
About this article
Cite this article
Pollizzi, K., Sun, IH., Patel, C. et al. Asymmetric inheritance of mTORC1 kinase activity during division dictates CD8+ T cell differentiation. Nat Immunol 17, 704–711 (2016). https://doi.org/10.1038/ni.3438
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3438
This article is cited by
-
Immunometabolic rewiring in tumorigenesis and anti-tumor immunotherapy
Molecular Cancer (2022)
-
Distinct strengths of mTORC1 control T-cell memory via transcriptional FOXO1 and metabolic AMPKα1 pathways in linear cell differentiation and asymmetric cell division models
Cellular & Molecular Immunology (2022)
-
The precursors of CD8+ tissue resident memory T cells: from lymphoid organs to infected tissues
Nature Reviews Immunology (2022)
-
The fellowship of regulatory and tissue-resident memory cells
Mucosal Immunology (2022)
-
Asymmetric cell division shapes naive and virtual memory T-cell immunity during ageing
Nature Communications (2021)