Abstract
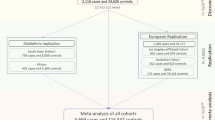
Central corneal thickness (CCT) is associated with eye conditions including keratoconus and glaucoma. We performed a meta-analysis on >20,000 individuals in European and Asian populations that identified 16 new loci associated with CCT at genome-wide significance (P < 5 × 10−8). We further showed that 2 CCT-associated loci, FOXO1 and FNDC3B, conferred relatively large risks for keratoconus in 2 cohorts with 874 cases and 6,085 controls (rs2721051 near FOXO1 had odds ratio (OR) = 1.62, 95% confidence interval (CI) = 1.4–1.88, P = 2.7 × 10−10, and rs4894535 in FNDC3B had OR = 1.47, 95% CI = 1.29–1.68, P = 4.9 × 10−9). FNDC3B was also associated with primary open-angle glaucoma (P = 5.6 × 10−4; tested in 3 cohorts with 2,979 cases and 7,399 controls). Further analyses implicate the collagen and extracellular matrix pathways in the regulation of CCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Dimasi, D.P., Burdon, K.P. & Craig, J.E. The genetics of central corneal thickness. Br. J. Ophthalmol. 94, 971–976 (2010).
Pedersen, U. & Bramsen, T. Central corneal thickness in osteogenesis imperfecta and otosclerosis. ORL J. Otorhinolaryngol. Relat. Spec. 46, 38–41 (1984).
Evereklioglu, C. et al. Central corneal thickness is lower in osteogenesis imperfecta and negatively correlates-vith the presence of blue sclera. Ophthalmic Physiol. Opt. 22, 511–515 (2002).
Cohen, E.J. Keratoconus and normal-tension glaucoma: a study of the possible association with abnormal biomechanical properties as measured by corneal hysteresis (An AOS Thesis). Trans Am. Ophthalmol. Soc. 107, 282–99 (2009).
Gordon, M.O. et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch. Ophthalmol. 120, 714–720, discussion 829–830 (2002).
Cornes, B.K. et al. Identification of four novel variants that influence central corneal thickness in multi-ethnic Asian populations. Hum. Mol. Genet. 21, 437–445 (2012).
Lu, Y. et al. Common genetic variants near the Brittle Cornea Syndrome locus ZNF469 influence the blinding disease risk factor central corneal thickness. PLoS Genet. 6, e1000947 (2010).
Vitart, V. et al. New loci associated with central cornea thickness include COL5A1, AKAP13 and AVGR8. Hum. Mol. Genet. 19, 4304–4311 (2010).
Vithana, E.N. et al. Collagen-related genes influence the glaucoma risk factor, central corneal thickness. Hum. Mol. Genet. 20, 649–658 (2011).
Abu, A. et al. Deleterious mutations in the zinc-finger 469 gene cause brittle cornea syndrome. Am. J. Hum. Genet. 82, 1217–1222 (2008).
Christensen, A.E. et al. Brittle cornea syndrome associated with a missense mutation in the zinc-finger 469 gene. Invest. Ophthalmol. Vis. Sci. 51, 47–52 (2010).
Khan, A.O., Aldahmesh, M.A., Mohamed, J.N. & Alkuraya, F.S. Blue sclera with and without corneal fragility (brittle cornea syndrome) in a consanguineous family harboring ZNF469 mutation (p.E1392X). Arch. Ophthalmol. 128, 1376–1379 (2010).
Segev, F. et al. Structural abnormalities of the cornea and lid resulting from collagen V mutations. Invest. Ophthalmol. Vis. Sci. 47, 565–573 (2006).
Gottsch, J.D. et al. Inheritance of a novel COL8A2 mutation defines a distinct early-onset subtype of fuchs corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 46, 1934–1939 (2005).
Biswas, S. et al. Missense mutations in COL8A2, the gene encoding the α2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum. Mol. Genet. 10, 2415–2423 (2001).
Kennedy, R.H., Bourne, W.M. & Dyer, J.A. A 48-year clinical and epidemiologic study of keratoconus. Am. J. Ophthalmol. 101, 267–273 (1986).
Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 42, 297–319 (1998).
Burdon, K.P. et al. Association of polymorphisms in the hepatocyte growth factor gene promoter with keratoconus. Invest. Ophthalmol. Vis. Sci. 52, 8514–8519 (2011).
Li, X. et al. A genome-wide association study identifies a potential novel gene locus for keratoconus, one of the commonest causes for corneal transplantation in developed countries. Hum. Mol. Genet. 21, 421–429 (2012).
Quigley, H.A. & Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 90, 262–267 (2006).
Stone, E.M. et al. Identification of a gene that causes primary open angle glaucoma. Science 275, 668–670 (1997).
Pasutto, F. et al. Heterozygous NTF4 mutations impairing neurotrophin-4 signaling in patients with primary open-angle glaucoma. Am. J. Hum. Genet. 85, 447–456 (2009).
Thorleifsson, G. et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat. Genet. 42, 906–909 (2010).
Burdon, K.P. et al. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat. Genet. 43, 574–578 (2011).
Wiggs, J.L. et al. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. PLoS Genet. 8, e1002654 (2012).
Ramdas, W.D. et al. Common genetic variants associated with open-angle glaucoma. Hum. Mol. Genet. 20, 2464–2471 (2011).
van Koolwijk, L.M. et al. Common genetic determinants of intraocular pressure and primary open-angle glaucoma. PLoS Genet. 8, e1002611 (2012).
Yang, J. et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat. Genet. 44, 369–375 (2012).
Medland, S.E. et al. Common variants in the trichohyalin gene are associated with straight hair in Europeans. Am. J. Hum. Genet. 85, 750–755 (2009).
Herndon, L.W. et al. Central corneal thickness in normal, glaucomatous, and ocular hypertensive eyes. Arch. Ophthalmol. 115, 1137–1141 (1997).
Harasymowycz, P.J., Papamatheakis, D.G., Ennis, M., Brady, M. & Gordon, K.D. Relationship between travoprost and central corneal thickness in ocular hypertension and open-angle glaucoma. Cornea 26, 34–41 (2007).
Liu, J.Z. et al. A versatile gene-based test for genome-wide association studies. Am. J. Hum. Genet. 87, 139–145 (2010).
Segrè, A.V., Groop, L., Mootha, V.K., Daly, M.J. & Altshuler, D. Common inherited variation in mitochondrial genes is not enriched for associations with type 2 diabetes or related glycemic traits. PLoS Genet. 6, pii: e1001058 (2010).
Han, S. et al. Association of variants in FRAP1 and PDGFRA with corneal curvature in Asian populations from Singapore. Hum. Mol. Genet. 20, 3693–3698 (2011).
Raychaudhuri, S. et al. Identifying relationships among genomic disease regions: predicting genes at pathogenic SNP associations and rare deletions. PLoS Genet. 5, e1000534 (2009).
Burkitt Wright, E.M. et al. Mutations in PRDM5 in brittle cornea syndrome identify a pathway regulating extracellular matrix development and maintenance. Am. J. Hum. Genet. 88, 767–777 (2011).
Souzeau, E. et al. The Australian and New Zealand Registry of Advanced Glaucoma: methodology and recruitment. Clin. Experiment. Ophthalmol. 40, 569–575 (2012).
Wiggs, J.L. et al. The NEIGHBOR Consortium Primary Open-Angle Glaucoma Genome-wide Association Study: rationale, study design, and clinical variables. J. Glaucoma published online, doi:10.1097/IJG.0b013e31824d4fd8 (23 July 2012).
Wiggs, J.L. et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Hum. Mol. Genet. 20, 4707–4713 (2011).
Lively, G.D. et al. Genetic dependence of central corneal thickness among inbred strains of mice. Invest. Ophthalmol. Vis. Sci. 51, 160–171 (2010).
Ramirez-Miranda, A., Nakatsu, M.N., Zarei-Ghanavati, S., Nguyen, C.V. & Deng, S.X. Keratin 13 is a more specific marker of conjunctival epithelium than keratin 19. Mol. Vis. 17, 1652–1661 (2011).
Cooper, L.J. et al. The role of dermatopontin in the stromal organization of the cornea. Invest. Ophthalmol. Vis. Sci. 47, 3303–3310 (2006).
Hayashida, Y. et al. Matrix morphogenesis in cornea is mediated by the modification of keratan sulfate by GlcNAc 6-O-sulfotransferase. Proc. Natl. Acad. Sci. USA 103, 13333–13338 (2006).
Mao, M., Hedberg-Buenz, A., Koehn, D., John, S.W. & Anderson, M.G. Anterior segment dysgenesis and early-onset glaucoma in nee mice with mutation of Sh3pxd2b. Invest. Ophthalmol. Vis. Sci. 52, 2679–2688 (2011).
Weaving, L. et al. Twist2: role in corneal stromal keratocyte proliferation and corneal thickness. Invest. Ophthalmol. Vis. Sci. 51, 5561–5570 (2010).
Zeller, T. et al. Genetics and beyond—the transcriptome of human monocytes and disease susceptibility. PLoS ONE 5, e10693 (2010).
Kilpeläinen, T.O. et al. Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nat. Genet. 43, 753–760 (2011).
Göring, H.H., Terwilliger, J.D. & Blangero, J. Large upward bias in estimation of locus-specific effects from genomewide scans. Am. J. Hum. Genet. 69, 1357–1369 (2001).
Visscher, P.M., Brown, M.A., McCarthy, M.I. & Yang, J. Five years of GWAS discovery. Am. J. Hum. Genet. 90, 7–24 (2012).
Willer, C.J., Li, Y. & Abecasis, G.R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Lango Allen, H. et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 467, 832–838 (2010).
Purcell, S.M. et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460, 748–752 (2009).
Painter, J.N. et al. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat. Genet. 43, 51–54 (2011).
Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 (2001).
Acknowledgements
A list of acknowledgments by study is provided in the Supplementary Note.
Author information
Authors and Affiliations
Consortia
Contributions
S.M., V.V., D.A.M., T.Y.W. and Y.L. conceived and designed the study, and liaised with the International Glaucoma Genetics Consortium for this project. Y.L. performed the primary analyses. S.M., J.Y., M.U., X.L., C.C.K., E.N.V., T.A., K.P.B., G.T., F.J., V.V., O.P., D.Y.L.L., L.J.C., C.C.Y.T., R.C., D.K., W.A., W.D.R., V.J.M.V., H.S., J.G., A.J.C., A. MacLeod, S.E., P.G.H., Y.B. and X.L. contributed to analysis. S.M. and Y.L. performed pathway analysis. J.E.C., P.M., U.T., A.F.W., N.P., C.P.P., M.G.A., J.L.W., M.A.H., L.R.P., C.E.W., N.G.M., D.A.M., C.M.v.D., T.Y.W., A.J.L., C.J.H. and Y.S.R. were the overseeing principal investigators of the individual studies. J.E.C., K.P.B., D.P.D., R.A.M., G.T., K.S., F.J., U.T., A.F.W., V.V., I.R., Z.V., C.H., O.P., H.C., J.F.W., B.F., N.P., A. Mirshahi, T.Z., R.H., F.G., R.C., K.J.L., C.P.P., D.Y.L.L., L.J.C., C.C.Y.T., M.G.A., D.K., J.L.W., L.R.P., M.U., J. Liu, B.L.Y., A.B.O., J.E.R., S.E.M., J.L.H., J.H.K., L.R.P., R.R.A., A.A.-K., J.L.W., M.A.H., N.G.M., Y.L., G.W.M., S.M., D.A.M., A.W.H., J.M., W.A., S.Y., C.P., T.L.Y., W.D.R., V.J.M.V., R.W., H.S., C.C.W.K., C.M.v.D., C.C.K., E.N.V., B.K.C., W.-T.T., E.S.T., C.-Y.C., J.-N.F., J. Li, S.M.S., T.A., T.Y.W., J.G., A.J.C., A. MacLeod, S.E., A.J.L., P.G.H., T.D.S., T.L.Y. and C.J.H. contributed reagents or methods to the genotyping, phenotyping and data analysis of corneal thickness data sets. J.E.C., K.P.B., D.P.D., R.A.M., C.P.P., D.Y.L.L., L.J.C., C.C.Y.T., J.L.W., L.R.P., M.U., J. Li, B.L.Y., A.B.O., J.E.R., S.E.M., J.L.H., J.H.K., L.R.P., R.R.A., A.A.-K., J.L.W., M.A.H., C.E.W., A.J.L., J.G., A.J.C., A. MacLeod, S.E., Y.S.R., Y.B., X.L., D.S., K.D.T., J.J.W., A.C.V. and J.I.R. contributed reagents or the genotyping, phenotyping and data analysis of the glaucoma, and keratoconus samples. Y.L. and S.M. wrote the first draft of this manuscript. K.P.B., V.V., C.C.K., Y.B., A. Mirshahi, A.W.H., D.K., P.G.H., W.D.R., J.L.W., C.M.v.D., Y.S.R., D.A.M., J.E.C. and T.Y.W. provided critical comments for manuscript revision. All authors reviewed the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
A list of members is provided in the Supplementary Note.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–13, Supplementary Figures 1–4 and Supplementary Note (PDF 1787 kb)
Rights and permissions
About this article
Cite this article
Lu, Y., Vitart, V., Burdon, K. et al. Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat Genet 45, 155–163 (2013). https://doi.org/10.1038/ng.2506
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2506
This article is cited by
-
Genetic variants in the FOXO1 and ZNF469 genes are associated with keratoconus in Sweden: a case-control study
BMC Ophthalmology (2024)
-
Genetic prescreening of a candidate for laser refractive surgery identifies risk for inadequate tissue response: a case report
Journal of Medical Case Reports (2022)
-
Evaluating the association between MPDZ-NF1B rs1324183 and keratoconus in an independent northwestern Chinese population
BMC Ophthalmology (2022)
-
TIPARP is involved in the regulation of intraocular pressure
Communications Biology (2022)
-
Single-cell atlas of keratoconus corneas revealed aberrant transcriptional signatures and implicated mechanical stretch as a trigger for keratoconus pathogenesis
Cell Discovery (2022)