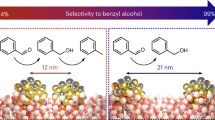
Abstract
A combination of the advantages of homogeneous and heterogeneous catalysis could enable the development of sustainable catalysts with novel reactivity and selectivity. Although heterogeneous catalysts are often recycled more easily than their homogeneous counterparts, they can be difficult to apply in traditional organic reactions and modification of their properties towards a desired reactivity is, at best, complex. In contrast, tuning the properties of homogeneous catalysts by, for example, modifying the ligands that coordinate a metal centre is better understood. Here, using olefin cyclopropanation reactions catalysed by dendrimer-encapsulated Au nanoclusters as examples, we demonstrate that changing the dendrimer properties allows the catalytic reactivity to be tuned in a similar fashion to ligand modification in a homogeneous catalyst. Furthermore, we show that these heterogeneous catalysts employed in a fixed-bed flow reactor allow fine control over the residence time of the reactants and thus enables the control over product distribution in a way that is not easily available for homogeneous catalysts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Astruc, D., Lu, F. & Aranzaes, J. R. Nanoparticles as recyclable catalysts. The frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 44, 7852–7872 (2005).
Bäckvall, J. E. International Symposium on Relations between Homogeneous and Heterogeneous Catalysis (Topics in Catalysis 53, Springer, 2010).
Thomas, J. M., Raja, R. & Lewis, D. W. Single site heterogeneous catalysis. Angew. Chem. Int. Ed. 44, 6456–6482 (2005).
Copéret, C., Chabanas, M., Saint-Arroman, R. P. & Basset, J. M. Homogeneous and heterogeneous catalysis: bridging the gap through surface organometallic chemistry. Angew. Chem. Int. Ed. 42, 156–181 (2003).
Chabanas, M., Baudouin, A., Copéret, C. & Basset, J. M. A highly active well-defined rhenium heterogeneous catalyst for olefin metathesis prepared via surface organometallic chemistry. J. Am. Chem. Soc. 123, 2062–2063 (2001).
Dufaud, V. & Basset J. M. Catalytic hydrogenolysis at low temperature and pressure of polyethylene and polypropylene to diesels or lower alkanes by a zirconium hydride supported on silica–alumina: a step toward polyolefin degradation by the microscopic reverse of Ziegler–Natta polymerization. Angew. Chem. Int. Ed. 37, 806–810 (1998).
Patil, N. T. Heterogeneous π-acid catalysis with metal nanoparticles. ChemCatChem 3, 1121–1125 (2011).
de Almeida, M. P. & Carabineiro, S. A. C. The best of two worlds from the gold catalysis universe: Making homogeneous heterogeneous. ChemCatChem 4, 18–29 (2012).
Zhang, Y., Cui, X., Shi, F. & Deng, Y. Nano-gold catalysis in the fine chemical synthesis. Chem. Rev. 112, 2467–2505 (2012).
Cong, H. & Porco, J. A. Jr Chemical synthesis of complex molecules using nanoparticle catalysis. ACS Catal. 2, 65–70 (2012).
Efe, C., Lykakis, I. N. & Stratakis, M. Gold nanoparticles supported on TiO2 catalyse the cycloisomerisation/oxidative dimerisation of aryl propargyl ethers. Chem. Commun. 47, 803–805 (2011).
Zhang, X. & Corma, A. Supported gold(III) catalysts for highly efficient three-component coupling reactions. Angew. Chem. Int. Ed. 47, 4358–4361 (2008).
Grirrane, A., Corma, A. & Garcia, H. Gold-catalyzed synthesis of aromatic azo compounds from anilines and nitroaromatics. Science 322, 1661–1664 (2008).
Han, J., Liu, Y. & Guo, R. Facile synthesis of highly stable gold nanoparticles and their unexpected excellent catalytic activity for Suzuki–Miyaura cross-coupling reaction in water. J. Am. Chem. Soc. 131, 2060–2061 (2009).
Lykakis, I. N., Psyllaki, A. & Stratakis, M. Oxidative cycloaddition of 1,1,3,3-tetramethyldisiloxane to alkynes catalyzed by supported gold nanoparticles. J. Am. Chem. Soc. 133, 10426–10429 (2011).
Shimizu, K., Sato, R. & Satsuma, A. Direct C–C cross-coupling of secondary and primary alcohols catalyzed by a γ-alumina-supported silver subnanocluster. Angew. Chem. Int. Ed. 48, 3982–3986 (2009).
Kim, S. W., Son, S. U., Lee, S. I., Hyeon, T. & Chung, Y. K. Cobalt on mesoporous silica: the first heterogeneous Pauson–Khand catalyst. J. Am. Chem. Soc. 122, 1550–1551 (2000).
Shakeri, M., Tai, C. W., Göthelid, E., Oscarsson, S. & Bäckvall, J. E. Small Pd nanoparticles supported in large pores of mesocellular foam: an excellent catalyst for racemization of amines. Chem. Eur. J. 17, 13269–13273 (2011).
Huang, W. Y et al. Highly active heterogeneous palladium nanoparticle catalysts for homogeneous electrophilic reactions in solution and the utilization of a continuous flow reactor. J. Am. Chem. Soc. 132, 16771–16773 (2010).
Witham, C. A. et al. Converting homogeneous to heterogeneous in electrophilic catalysis using monodisperse metal nanoparticles. Nature Chem. 2, 36–41 (2010).
Li, Y. et al. A Pt-cluster-based heterogeneous catalyst for homogeneous catalytic reactions: X-ray absorption spectroscopy and reaction kinetic studies of their activity and stability against leaching. J. Am. Chem. Soc. 133, 13527–13533 (2011).
Gorin, D. J. & Toste, F. D. Relativistic effects in homogeneous gold catalysis. Nature 446, 395–403 (2007).
Kim, Y. G., Oh, S. K. & Crooks, R. M. Preparation and characterization of 1–2 nm dendrimer encapsulated gold nanoparticles having very narrow size distributions. Chem. Mater. 16, 167–172 (2004).
Crooks, R. M., Zhao, M. Q., Sun, L., Chechik, V. & Yeung, L. K. Dendrimer encapsulated metal nanoparticles: synthesis, characterization, and applications to catalysis. Acc. Chem. Res. 34, 181–190 (2001).
Huang, W. Y. et al. Dendrimer templated synthesis of one nanometer Rh and Pt particles supported on mesoporous silica: catalytic activity for ethylene and pyrrole hydrogenation. Nano Lett. 8, 2027–2034 (2008).
Sun, L. & Crooks, R. M. Dendrimer-mediated immobilization of catalytic nanoparticles on flat, solid supports. Langmuir 18, 8231–8236 (2002).
Johansson, M. J., Gorin, D. J., Staben, S. T. & Toste, F. D. Gold(I)-catalysed stereoselective olefin cyclopropanation. J. Am. Chem. Soc. 127, 18002–18003 (2005).
Peng, S. et al. A facile synthesis of monodisperse Au nanoparticles and their catalysis of CO oxidation. Nano Res. 1, 229–234 (2008).
Borodko, Y. et al. Spectroscopic study of platinum and rhodium dendrimer (PAMAM G4OH) compounds: structure and stability. J. Phys. Chem. C 115, 4757–4767 (2011).
Kung, H. H. & Kung, M. C. Effect of surface diffusion on the selectivity of catalytic reactions. Chem. Eng. Sci. 33, 1003–1008 (1978).
Sahoo, H. R., Kralj, J. G. & Jensen, K. F. Multistep continuous-flow microchemical synthesis involving multiple reactions and separations. Angew. Chem. Int. Ed. 46, 5704–5708 (2007).
Noel, T., Kuhn, S., Musacchio, A. J., Jensen, K. F. & Buchwald, S. L. Suzuki–Miyaura cross-coupling reactions in flow: multistep synthesis enabled by a microfluidic extraction. Angew. Chem. Int. Ed. 50, 5943–5946 (2011).
Webb, D. & Jamison, T. F. Continuous flow multi-step organic synthesis. Chem. Sci. 1, 675–680 (2010).
Nagaki, A. et al. Lithiation of 1,2-dichloroethene in flow microreactors: versatile synthesis of alkenes and alkynes by precise residence-time control. Angew. Chem. Int. Ed. 51, 3245–3248 (2012).
Gorin, D. J., Watson, I. D. G. & Toste, F. D. Fluorenes and styrenes by Au(I)-catalyzed annulation of enynes and alkynes. J. Am. Chem. Soc. 130, 3736–3737 (2008).
Acknowledgements
We acknowledge support from the Director, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geological and Biosciences of the US Department of Energy (DOE) under contract DE-AC02-05CH11231. Nanoparticle TEM imaging was performed by S. Alayoglu at the Molecular Foundry Imaging Facility, Lawrence Berkeley National Laboratory, which is supported by the Office of Science, Office of Basic Energy Sciences of the US DOE under contract DE-AC02-05CH11231.
Author information
Authors and Affiliations
Contributions
E.G. and J.H.L. performed the experiments and synthesized materials, substrates and catalysts. F.D.T. and G.A.S. supervised the research. All authors contributed to the conception of the experiments, discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 759 kb)
Rights and permissions
About this article
Cite this article
Gross, E., Liu, JC., Toste, F. et al. Control of selectivity in heterogeneous catalysis by tuning nanoparticle properties and reactor residence time. Nature Chem 4, 947–952 (2012). https://doi.org/10.1038/nchem.1465
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1465
This article is cited by
-
Controlling hot electron flux and catalytic selectivity with nanoscale metal-oxide interfaces
Nature Communications (2021)
-
Comparative Study of Homogeneous and Silica Immobilized N^N and N^O Palladium(II) Complexes as Catalysts for Hydrogenation of Alkenes, Alkynes and Functionalized Benzenes
Catalysis Letters (2020)
-
Magnetic mesoporous poly-melamine–formaldehyde: an efficient and recyclable catalyst for straightforward one-pot synthesis of imidazo[1,2-a]pyridines
Journal of the Iranian Chemical Society (2019)
-
Surface Science Approach to the Molecular Level Integration of the Principles in Heterogeneous, Homogeneous, and Enzymatic Catalysis
Topics in Catalysis (2018)
-
Sintering-Resistant Nanoparticles in Wide-Mouthed Compartments for Sustained Catalytic Performance
Scientific Reports (2017)