Abstract
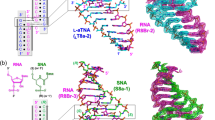
The ruthenium complex [Ru(phen)2(dppz)]2+ (where phen is phenanthroline and dppz dipyridophenazine is known as a ‘light switch’ complex because its luminescence in solution is significantly enhanced in the presence of DNA. This property is poised to serve in diagnostic and therapeutic applications, but its binding mode with DNA needs to be elucidated further. Here, we describe the crystal structures of the Λ enantiomer bound to two oligonucleotide duplexes. The dppz ligand intercalates symmetrically and perpendicularly from the minor groove of the d(CCGGTACCGG)2 duplex at the central TA/TA step, but not at the central AT/AT step of d(CCGGATCCGG)2. In both structures, however, a second ruthenium complex links the duplexes through the combination of a shallower angled intercalation into the C1C2/G9G10 step at the end of the duplex, and semi-intercalation into the G3G4 step of an adjacent duplex. The TA/TA specificity of the perpendicular intercalation arises from the packing of phenanthroline ligands against the adenosine residue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lerman, L. S. Structural considerations in the interaction of deoxyribonucleic acid and acridines. J. Mol. Biol. 3, 18–30 (1961).
Shieh, H.-S., Berman, H. M., Dabrow, M. & Neidle, S. The structure of a drug–deoxydinucleoside phosphate complex; generalized conformational behaviour of intercalation complexes with RNA and DNA fragments. Nucleic Acids Res. 8, 85–98 (1980).
Neidle, S. The molecular basis for the action of some DNA-binding drugs. Prog. Med. Chem. 16, 151–221 (1979).
Todd, A. K. et al. Major groove binding and ‘DNA-induced’ fit in the intercalation of a derivative of the mixed topoisomerase I/II poison N-(2-(dimethylamino)ethyl)acridine-4-carboxamide (DACA) into DNA: X-ray structure complexed to d(CG(5-BrU)ACG)2 at 1.3 Å resolution. J. Med. Chem. 42, 536–540 (1999).
Hopcroft, N. H., Brogden, A. L., Searcey, M. & Cardin, C. J. X-ray crystallographic study of DNA duplex cross-linking: simultaneous binding to two d(CGTACG)2 molecules by a bis(9-aminoacridine-4-carboxamide) derivative. Nucleic Acids Res. 34, 6663–6672 (2006).
Brogden, A. L., Hopcroft, N. H., Searcey, M. & Cardin, C. J. Ligand bridging of the DNA Holliday junction: molecular recognition of a stacked-X four-way junction by a small molecule. Angew. Chem. Int. Ed. 119, 3924–3928 (2007).
Frederick, C. A. et al. Structural comparison of anticancer drug–DNA complexes: adriamycin and daunomycin. Biochemistry 29, 2538–2549 (1990).
Hou, M.-H., Robinson, H., Gao, Y.-G. & Wang, A. H.-J. Crystal structure of actinomycin D bound to the CTG triplet repeat sequence linked to neurological diseases. Nucleic Acids Res. 30, 4910–4917 (2002).
Garbett, N. C. & Chaires, J. B. Biophysical tools for biologists: vol. 1 in vitro techniques. Methods Cell Biol. 84, 3–23 (2008).
Wheate, N. J., Brodie, C. R., Collins, J. G., Kemp, S. & Aldrich-Wright, J. R. DNA intercalators in cancer therapy: organic and inorganic drugs and their spectroscopic tools of analysis. Mini Rev. Med. Chem. 7, 627–648 (2007).
Dai, J., Punchihewa, C., Mistry, P., Ooi, A. T. & Yang, D. Novel DNA bis-intercalation by MLN944, a potent clinical bisphenazine anticancer drug. J. Biol. Chem. 279, 46096–46103 (2004).
Zeglis, B. M., Pierre, V. C. & Barton, J. K. Metallo-intercalators and metallo-insertors. Chem. Commun. 44, 4549–4696 (2007).
Liu, H.-K. & Sadler, P. J. Metal complexes as DNA intercalators. Acc. Chem. Res. 44, 349–359 (2011).
Boer, D. R., Canals, A. & Coll, M. DNA-binding drugs caught in action: the latest 3D pictures of drug–DNA complexes. Dalton Trans. 3, 399–414 (2009).
Chambron, J. C., Sauvage, J. P., Amouyal, E. & Koffi, P. Ru(bipy)2(dypyridophenazine)2+: a complex with a long range directed charge transfer excited state. Nouv. J. Chim. 9, 527–529 (1985).
McKinley, A. W., Lincoln, P. & Tuite, E. M. Environmental effects on the photophysics of transition metal complexes with dipyrido[2,3-1:3′, 2′-c]phenazine (dppz) and related ligands. Coord. Chem. Rev. 255, 2676–2692 (2011).
Friedman, A. E. et al. A molecular light switch for DNA: Ru(bpy)2(dppz)2+ . J. Am. Chem. Soc. 112, 4960–4962 (1990).
Hiort, C., Lincoln, P. & Norden, B. DNA binding of Δ- and Λ-Ru(phen)2dppz]2+. J. Am. Chem. Soc. 115, 3448–3454 (1993).
Smith, J. A., George, M. W. & Kelly, J. M. Transient spectroscopy of dipyridophenazine metal complexes which undergo photo-induced electron transfer with DNA. Coord. Chem. Rev. 255, 2666–2675 (2011).
Elias, B. et al. Photooxidation of guanine by a ruthenium dipyridophenazine complex intercalated in a double-stranded polynucleotide monitored directly by picosecond visible and infrared transient absorption spectroscopy. Chem. Eur J. 14, 369–375 (2007).
Barton, J. K., Olmon, E. D. & Sontz, P. A. Metal complexes for DNA-mediated charge transport. Coord. Chem. Rev. 255, 619–634 (2011).
Hartshorn, R. M. & Barton, J. K. Novel dipyridophenazine complexes of ruthenium(II): exploring luminescent reporters of DNA. J. Am. Chem. Soc. 114, 5919–5925 (1992).
Lincoln, P., Broo, A. & Nordén, B. Diastereomeric DNA-binding geometries of intercalating ruthenium (II) trischelates probed by linear dichroism: [Ru(phen)2dppz]2+ and [Ru(phen)2bdppz]2+. J. Am. Chem. Soc. 118, 2644–2653 (1996).
Haq, I. et al. Interaction of Δ- and Λ-Ru(phen)2dppz]2+ with DNA: a calorimetric and equilibrium binding study. J. Am. Chem. Soc. 117, 4788–4796 (1995).
Hall, J. P. et al. Structure determination of an intercalation ruthenium dipyridophenazine complex which kinks DNA by semiintercalation of a tetraazaphenanthrene ligand. Proc. Natl Acad. Sci. USA 108, 17610–17614 (2011).
Tuite, E., Lincoln, P. & Nordén, B. Photophysical evidence that Δ- and Λ-[Ru(phen)2(dppz)]2+ intercalate DNA from the minor groove. J. Am. Chem. Soc. 119, 239–240 (1997).
Thorpe, J. H., Teixeira, S. C. M., Gale, B. C. & Cardin, C. J. Structural characterization of a new crystal form of the four-way Holliday junction formed by the DNA sequence d(CCGGTACCGG)2: sequence versus lattice? Acta Crystallogr. D 58, 567–569 (2002).
Eichman, B. F., Vargason, J. M., Mooers, B. H. M. & Shing Ho, P. The Holliday junction in an inverted repeated DNA sequence: sequence effects on the structure of four-way junctions. Proc. Natl Acad. Sci. USA 97, 3971–3976 (2000).
Hays, F. A. et al. How sequence defines structure: a crystallographic map of DNA structure and conformation. Proc. Natl Acad. Sci. USA 102, 7157–7162 (2005).
Neidle, S. Principles of Nucleic Acid Structure 144–158 (Academic Press, 2007).
Pierre, V. C., Kaiser, J. T. & Barton, J. K. Insights into finding a mismatch through the structure of a mispaired DNA bound by a rhodium intercalator. Proc. Natl Acad. Sci. USA 104, 429–434 (2007).
Brennaman, M. K., Meyer, T. J. & Papanikolas, J. M. [Ru(bpy)2dppz]2+ light-switch mechanism in protic solvents as studies through temperature-dependent lifetime measurements. J. Phys. Chem. A 108, 9938–9944 (2004).
Önfeld, T., Olofsson, J., Lincoln, P. & Nordén, B. Picosescond and steady state emission of [Ru(phen)2dppz]2+ in glycerol: anomalous temperature dependence. J. Phys. Chem A 107, 1000–1009 (2003).
Olson, E. J. C. et al. First observation of the key intermediate in the ‘light-switch’ mechanism of [Ru(phen)2dppz]2+. J. Am. Chem. Soc. 119, 11458–11467 (1997).
Lincoln, P. A generalised McGhee–von Hippel method for the cooperative binding of different competing ligands to an infinite one-dimensional lattice. Chem. Phys. Lett. 288, 647–656 (1998).
Winter, G. Xia2: an expert system for macromolecular crystallography data reduction. J. Appl. Crystallogr. 43, 186–190 (2010).
Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 26, 795–800 (1993).
Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D 62, 72–82 (2006).
Emsley, P., Lohkamp, B., Scott, W. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 (1997).
Collaborative computational project, number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. D 64, 113–122 (2008).
Smith, C. K., Davies, G. J., Dodson. E. J. & Moore, M. H. DNA–nogalamycin interactions: the crystal structure of d(TGATCA) complexed with nogalamycin. Biochemistry 34, 415–425 (1995).
Acknowledgements
The authors thank G. Doorley (formerly of Trinity College Dublin) for preparing the ruthenium complex, and P. Lincoln (Chalmers University, Gothenburg, Sweden) for reading a draft of the manuscript, very helpful discussions, and making available his unpublished observations. H.N. is funded by a joint ILL/ESRF studentship and the Chemistry Department, University of Reading. J.P.H. is funded by Diamond Light Source and a University of Reading University studentship. The authors acknowledge additional financial support from the Royal Society, the Royal Irish Academy and the Science Foundation Ireland (grant 06/RF/CHP035).
Author information
Authors and Affiliations
Contributions
C.J.C., J.P.H. and J.M.K. conceived and designed the experiments. H.N., J.P.H., K.O'S. and C.J.C. performed the experiments. G.W. and T.S. contributed analytical tools. J.P.H., H.N. and C.J.C. analysed the data. C.J.C. wrote the paper, with contributions from J.M.K., J.P.H. and H.N. All authors discussed the results and commented on the manuscript. H.N. and J.P.H. contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 593 kb)
Rights and permissions
About this article
Cite this article
Niyazi, H., Hall, J., O'Sullivan, K. et al. Crystal structures of Λ-[Ru(phen)2dppz]2+ with oligonucleotides containing TA/TA and AT/AT steps show two intercalation modes. Nature Chem 4, 621–628 (2012). https://doi.org/10.1038/nchem.1397
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1397
This article is cited by
-
The interactions of monomeric acridines and unsymmetrical bisacridines (UAs) with DNA duplexes: an insight provided by NMR and MD studies
Scientific Reports (2023)
-
Enabling programmable dynamic DNA chemistry using small-molecule DNA binders
Nature Communications (2023)
-
Recognizing and stabilizing miR-21 by chiral ruthenium(II) complexes
BMC Chemistry (2020)
-
A strong preference for the TA/TA dinucleotide step discovered for an acridine-based, potent antitumor dsDNA intercalator, C-1305: NMR-driven structural and sequence-specificity studies
Scientific Reports (2020)
-
Anticancer activity, DNA binding and cell mechanistic studies of estrogen-functionalised Cu(II) complexes
JBIC Journal of Biological Inorganic Chemistry (2020)