Abstract
Ferroptosis is a non-apoptotic form of cell death induced by small molecules in specific tumour types, and in engineered cells overexpressing oncogenic RAS. Yet, its relevance in non-transformed cells and tissues is unexplored and remains enigmatic. Here, we provide direct genetic evidence that the knockout of glutathione peroxidase 4 (Gpx4) causes cell death in a pathologically relevant form of ferroptosis. Using inducible Gpx4−/− mice, we elucidate an essential role for the glutathione/Gpx4 axis in preventing lipid-oxidation-induced acute renal failure and associated death. We furthermore systematically evaluated a library of small molecules for possible ferroptosis inhibitors, leading to the discovery of a potent spiroquinoxalinamine derivative called Liproxstatin-1, which is able to suppress ferroptosis in cells, in Gpx4−/− mice, and in a pre-clinical model of ischaemia/reperfusion-induced hepatic damage. In sum, we demonstrate that ferroptosis is a pervasive and dynamic form of cell death, which, when impeded, promises substantial cytoprotection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Galluzzi, L. et al. Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 19, 107–120 (2012).
Ofengeim, D. & Yuan, J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat. Rev. Mol. Cell Biol. 14, 727–736 (2013).
Fulda, S. & Vucic, D. Targeting IAP proteins for therapeutic intervention in cancer. Nat. Rev. Drug Discov. 11, 109–124 (2012).
Vanden Berghe, T., Linkermann, A., Jouan-Lanhouet, S., Walczak, H. & Vandenabeele, P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 15, 135–147 (2014).
Vandenabeele, P., Galluzzi, L., Vanden Berghe, T. & Kroemer, G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 11, 700–714 (2010).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Dixon, S. J. & Stockwell, B. R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 10, 9–17 (2013).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Skouta, R. et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 136, 4551–4556 (2014).
Conrad, M. & Sato, H. The oxidative stress-inducible cystine/glutamate antiporter, system x (c) (-): cystine supplier and beyond. Amino Acids 42, 231–246 (2012).
Seiler, A. et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 8, 237–248 (2008).
Sun, D. & Funk, C. D. Disruption of 12/15-lipoxygenase expression in peritoneal macrophages. Enhanced utilization of the 5-lipoxygenase pathway and diminished oxidation of low density lipoprotein. J. Biol. Chem. 271, 24055–24062 (1996).
Gilbert, N. C. et al. Conversion of human 5-lipoxygenase to a 15-lipoxygenase by a point mutation to mimic phosphorylation at Serine-663. FASEB J. 26, 3222–3229 (2012).
Mannes, A. M., Seiler, A., Bosello, V., Maiorino, M. & Conrad, M. Cysteine mutant of mammalian GPx4 rescues cell death induced by disruption of the wild-type selenoenzyme. FASEB J. 25, 2135–2144 (2011).
Takahashi, N. et al. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Disease 3, e437 (2012).
Conrad, M. et al. 12/15-lipoxygenase-derived lipid peroxides control receptor tyrosine kinase signaling through oxidation of protein tyrosine phosphatases. Proc. Natl Acad. Sci. USA 107, 15774–15779 (2010).
Kagan, V. E. et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 1, 223–232 (2005).
Cocheme, H. M. et al. Mitochondrial targeting of quinones: therapeutic implications. Mitochondrion 7 (suppl.), S94–S102 (2007).
Kriska, T., Pilat, A., Schmitt, J. C. & Girotti, A. W. Sterol carrier protein-2 (SCP-2) involvement in cholesterol hydroperoxide cytotoxicity as revealed by SCP-2 inhibitor effects. J. Lipid Res. 51, 3174–3184 (2010).
Vila, A., Levchenko, V. V., Korytowski, W. & Girotti, A. W. Sterol carrier protein-2-facilitated intermembrane transfer of cholesterol- and phospholipid-derived hydroperoxides. Biochemistry 43, 12592–12605 (2004).
Kim, M. S., Wessely, V. & Lan, Q. Identification of mosquito sterol carrier protein-2 inhibitors. J. Lipid Res. 46, 650–657 (2005).
Tyurina, Y. Y. et al. Characterization of cardiolipins and their oxidation products by LC-MS analysis. Chem. Phys. Lipids 179, 3–10 (2014).
Nomura, K., Imai, H., Koumura, T., Kobayashi, T. & Nakagawa, Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem. J. 351, 183–193 (2000).
McIntyre, T. M. Bioactive oxidatively truncated phospholipids in inflammation and apoptosis: formation, targets, and inactivation. Biochim. Biophys. Acta 1818, 2456–2464 (2012).
Liang, H. et al. Short form glutathione peroxidase 4 is the essential isoform required for survival and somatic mitochondrial functions. J. Biol. Chem. 284, 30836–30844 (2009).
Wortmann, M. et al. Combined deficiency in glutathione peroxidase 4 and vitamin e causes multiorgan thrombus formation and early death in mice. Circ. Res. 113, 408–417 (2013).
Yant, L. J. et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic. Biol. Med. 34, 496–502 (2003).
Brigelius-Flohe, R. & Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 1830, 3289–3303 (2013).
Zarjou, A. et al. Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J. Clin. Invest. 123, 4423–4434 (2013).
Linkermann, A. et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc. Natl Acad. Sci. USA 110, 12024–12029 (2013).
Wang, C., Weerapana, E., Blewett, M. M. & Cravatt, B. F. A chemoproteomic platform to quantitatively map targets of lipid-derived electrophiles. Nat. Methods 11, 79–85 (2014).
Wong, W. W. et al. RIPK1 is not essential for TNFR1-induced activation of NF-κB. Cell Death Differ. 17, 482–487 (2010).
Schneider, M. et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 23, 3233–3242 (2009).
Hameyer, D. et al. Toxicity of ligand-dependent Cre recombinases and generation of a conditional Cre deleter mouse allowing mosaic recombination in peripheral tissues. Physiol. Genomics 31, 32–41 (2007).
Kiermayer, C., Conrad, M., Schneider, M., Schmidt, J. & Brielmeier, M. Optimization of spatiotemporal gene inactivation in mouse heart by oral application of tamoxifen citrate. Genesis 45, 11–16 (2007).
Parrinello, S. et al. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 5, 741–747 (2003).
Herbach, N. et al. Diabetic kidney lesions of GIPRdn transgenic mice: podocyte hypertrophy and thickening of the GBM precede glomerular hypertrophy and glomerulosclerosis. Am. J. Physiol. Renal. Physiol. 296, F819–F829 (2009).
Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal. Biochem. 27, 502–522 (1969).
Rouser, G., Fkeischer, S. & Yamamoto, A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5, 494–496 (1970).
Tyurina, Y. Y. et al. A mitochondrial pathway for biosynthesis of lipid mediators. Nat. Chem. 6, 542–552 (2014).
Tanaka, R., Hatate, H., Ito, M. & Nakamura, T. Elevation of lipid peroxide level and production of hydroxy lipids in cultured Hepa-T1 cells by oxidative stressors. Fisheries Sci. 72, 665–672 (2006).
Tyurina, Y. Y., Tyurin, V. A., Epperly, M. W., Greenberger, J. S. & Kagan, V. E. Oxidative lipidomics of gamma-irradiation-induced intestinal injury. Free Radic. Biol. Med. 44, 299–314 (2008).
Kim, J. & Hoppel, C. L. Monolysocardiolipin: improved preparation with high yield. J. Lipid Res. 52, 389–392 (2011).
Eggenhofer, E. et al. Unconventional RORgammat + T cells drive hepatic ischemia reperfusion injury. J. Immunol. 191, 480–487 (2013).
Acknowledgements
We are grateful to D. Green for his valuable comments and critical reading of the manuscript. We would also like to thank A. Berns, The Netherlands Cancer Institute, Amsterdam, for providing the ROSA26-CreERT2 mouse line, J. Silke (The Walter and Eliza Hall Institute, Melbourne, Australia) for the Rip1-knockout cells and M. P. Murphy (Medical Research Council, London, UK) for provision of MitoQ and Mito-BODIPY. We are most grateful to L. Pichl for excellent technical assistance. This work was in part supported by the Deutsche Forschungsgemeinschaft (DFG) CO 291/2-3 to M.C., a fellowship from Alexander von Humboldt-Stiftung to J.P.F.A., a fellowship from the Japan Society for the Promotion of Science (JSPS) to S.K., the Human Frontier Science Program (HFSP) RGP0013 to M.C. and V.E.K., HL114453 (NIH) to V.E.K., and by the m4 Award (Bavarian Ministry of Economic Affairs) to M.C. and J.A.S.
Author information
Authors and Affiliations
Contributions
J.P.F.A. and M.C. conceived the study. Electron microscopy studies were carried out and analysed by M.A. and A.W. Eicosanoid release and data analysis were performed by V.J.H. and V.B.O. Oxi-lipidomics analysis and interpretation was performed by Y.Y.T., V.A.T. and V.E.K. Optimized RSL3 synthesis was conceived and carried out by S.B.T. and B.R.S. Liver ischaemia/reperfusion experiments were performed and analysed by E.E. and E.K.G. Gpx4/Alox15 double-knockout generation and histopathological analysis was conceived and performed by G.W.B., N.H., H.B., F.N., I.E. and R.W. Small-molecule screen was conceived and analysed by B.P., J.A.S. and M.C. Experiments were performed by J.P.F.A., M.S., N.H., D.B., S.K., T.S., H.F., O.Y. and M.H. Evaluation and interpretation of the in vitro data was performed by J.P.F.A., O.R. and M.C. Paper was written by J.P.F.A., V.E.K. and M.C. All authors read and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare that a patent application has been filed for some of the compounds described in this work.
Integrated supplementary information
Supplementary Figure 2 Genetic loss of Alox15 does not rescue cell death and ARF induced by Gpx4 inactivation.
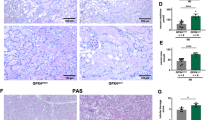
(a) TAM-inducible Gpx4 deletion in lung fibroblasts derived from CreERT2;Gpx4fl/fl/Alox15−/− mice (PZL cells). (b) PZ (Alox15-proficient) and PZL (Alox15-deficient) lung fibroblasts can be rescued from Gpx4 deletion-induced cell death by lipophilic (αToc), but not by hydrophilic antioxidants (NAC, N-acetylcysteine). Gpx4 knockout was induced by the addition of 1 μM TAM in the presence of increasing concentrations of the antioxidants; cell viability was measured 72 h later. (c,d) Knockdown of Alox5 delays cell death upon Gpx4 deletion only in cells additionally lacking Alox15 (PZL) (d), but not in cells proficient for Alox15 (PZ) (c). Cell death was monitored at different time points after KO induction using AquaBluerTM. Knockdown efficiency was monitored by quantitative PCR. Data shown represents the mean ± s.d. of n = 3 wells (b, c, d) of a 96-well plate (or 6-well plate for RNA isolation) from a representative experiment performed independently three times, ANOVA (∗p < 0.05). (e) Histological analysis of kidneys of TAM-treated CreERT2;Gpx4fl/fl/Alox15−/− mice revealed widespread tubular cell death, interstitial edema, and proteinaceous casts in distal tubules like TAM-treated CreERT2;Gpx4fl/fl/Alox15+/+ mice (scale bars 100 μm) (Periodic Acid-Schiff Stain, PAS). (f) Electron micrographs confirmed the severe destruction of renal tubule cells in symptomatic CreERT2;Gpx4fl/fl/Alox15+/+ mice (scale bars 10 μm). (g) Staining against F4/80, a 160 kD glycoprotein expressed by murine macrophages, revealed interstitial inflammatory cell infiltrates in response to massive tissue injury in symptomatic Gpx4 null kidneys (CreERT2;Gpx4fl/fl). Staining against active caspase-3 (a-Casp-3) showed faint staining in kidney tissue of Gpx4 knockout mice.
Supplementary Figure 3 Caspases are not involved in ferroptotic cell death.
(a) The pan caspase inhibitor Z-VAD-FMK (zvad) does not prevent cell death triggered by Gpx4 deletion. Knockout of Gpx4 was induced by adding 1 μM TAM in the presence of increasing zvad concentrations and cell viability was determined 72 h later. Data shown represents the mean ± s.d. of n = 3wells of a 96-well plate from a representative experiment performed independently three times. (b) Inducible Gpx4 deletion in Pfa-1 cells does not lead to caspase activation. Caspase activity was determined using CellEventTM Caspase-3/7 Green Detection Reagent (Life Technologies, Thermo Fisher Scientific) at different time points after KO induction. (c) Ferroptosis inducers do not increase caspase activity. Activities were determined in Pfa-1 cells after treatment with the different inducers at specific time points [TAM (1 μM – 48 h), BSO (10 μM – 48 h), erastin (1 μM – 12 h), RSL3 (0.5 μM – 3 h), staurosporine (Stauro; 0.2 μM – 6 h) and TNFα (10 ng ml−1 – 9 h)]. (d) Class I FINs (BSO and erastin) decrease GSH levels, whereas the class II FINs RSL3 and Gpx4 do not impact on GSH levels. Total GSH levels were determined after treatment with the different inducers at specific time points [BSO (10 μM – 24 h), erastin (1 μM – 8 h), RSL3 (0.5 μM – 3 h) TAM (1 μM – 48 h). Data shown (b, c, d) represents the mean ± s.d. of n = 3 wells of a 6-well plate from a representative experiment performed independently two (b, c) or three (d) times. (e) Kinetics of cell death induced by ferroptosis-inducing agents and apoptosis-inducing agents in Pfa1 cells. Cells were treated with BSO (20 μM), erastin (2 μM), RSL3 (0.5 μM), TAM (1 μM), Staurosporine (0.5 μM) and TNFα (10 ng ml−1). Cellular viability was assayed using AquaBluerTM. (f) Profile of rescue of Gpx4 proficient Pfa1 cells treated with apoptotic and ferroptotic cell death triggers. Cells were treated with the cell death inducers for the indicated times [TAM (1 μM – 72 h), erastin (1 μM – 24 h), RSL3 (0.5 μM – 24 h), TNFα (10 ng ml−1 – 48 h) and Staurosporine (0.2 μM – 48 h)]. The rescue profile was performed using fixed concentrations of Z-VAD-FMK (zvad, 50 μM), Nec1 (25 μM), αToc (2 μM), Fer1 (0.2 μM) and DFO (10 μM), erastin (1 μM – 12 h), RSL3 (0.5 μM – 3 h), Staurosporine (0.2 μM – 6 h) and TNFα (10 ng ml−1 – 9 h). (gh) Overexpression of active Gpx4 (WT), but not Gpx4-U46S in Gpx4-proficient Pfa1 cells, increases resistance to RSL3- and erastin-induced ferroptosis. Data shown represents the mean ± s.d. of n = 3 (e, f) or n = 4 wells (g, h) of a 96-well plate from a representative experiment performed independently three times.
Supplementary Figure 4 Receptor protein interacting kinases 1 and 3 do not contribute to ferroptotic cell death.
(a) An immunoblot against Rip3 showing that Rip3 is only weakly expressed in Pfa1 cells in contrast to L929 fibroblasts. (b) Rip1 knockout (KO) cells do not show increased resistance to the ferroptosis inducing agents as compared to Rip1 wildtype (WT) cells. Cell death was assessed 24 h or 48 h after treatment with erastin or BSO, respectively (top row). Nec1 prevents cell death induced by ferroptosis inducers even in Rip1 KO cells. Viability was assessed after adding increasing concentrations of Nec1 (0–50 μM) to Rip1 KO cells treated with 10 μM BSO or 0.4 μM erastin (bottom row). (c) Ultrastructural analysis of Gpx4-proficient Pfa-1 cells treated with various FINs, classical inducers of apoptotic cell death and on Gpx4 deletion (TAM) (Scale bars are 2 μm in the top row and 200 nm in the bottom row). (d) MitoQ and DecylQ (1 μM) prevented cell death induced by Gpx4 deletion, an effect that is not modulated by dissipating the mitochondrial membrane potential with trifluorocarbonylcyanide phenylhydrazone (FCCP, 1 μM). Data shown represents the mean ± s.d. of n = 4 wells (d) or n = 6 wells (b) of a 96-well plate from an experiment performed independently three times.
Supplementary Figure 5 Knockdown of Alox5 slows down the release of HETEs and delays cell death kinetics in Alox15/Gpx4 double knockout cells.
(a,b) Quantification of released arachidonic acid metabolites (5-HETE, 11-HETE, 12-HETE and 15-HETE) was performed by LC/MS/MS from supernatants collected at 24, 48 and 72 h after Gpx4 KO induction (PZ cells are wildtype for Alox15, whereas PZL cells are knockout for Alox15). Data shown represents the mean ± s.d. from one well of a 6-well plate, pooled from n = 3 repeated two times. (c) PZL cells stably transduced with an shRNA against Alox5 are less susceptible to ferroptotic stimuli than the parental PZL and PZ cells, whereas all the cell lines showed similar sensitivity to staurosporine-induced apoptosis and tBOOH-induced necrosis. (Data shown represents the mean ± s.d. from one well of a 96-well plate, pooled from n = 3 (c) representative experiment performed three times, ANOVA (∗p < 0.05). (d) siRNA-mediated knockdown of SCP2 partially protects against cell death in response to Gpx4 deletion. Shown are experiments performed with three independent siRNAs (Scp2_1, Scp2_2, Scp2_3) (Scb, scrambled siRNA). (e) Kinetics of cell death on Gpx4 deletion and SCP-2 knockdown (Scp2_2). Pfa1 cells were induced and transfection with the siRNA was performed 6 h thereafter, and viability was determined at different time points. Data shown represents the mean ± s.d. of n = 3 wells (e) or n = 4 wells (e) of a 6- (e) or 12- (e) well plate from an experiment performed independently three times, ANOVA (∗p < 0.05).
Supplementary Figure 6 Oxi-lipidomics of Gpx4−/− kidneys.
(a) MS2 fragmentation patterns of non-oxidized PC with m/z 816.537 (upper panel) and its oxygenated species with m/z 848.570 (lower panel). PCs were detected as acetate adducts. The levels of hydroperoxy PC species in kidney from Gpx4 null and wildtype mice. Hydroperoxy PC species (18:2/18:2–OOH, m/z 872.575), (18:0/18:2–OOH, m/z 876.606), (16:0/22:6–OOH, m/z 896.575) and (18:1/20:4–OOH, m/z 898.587) were originated from PC species (18:2/18:2, 840.576), (18:0/18:2, m/z 844.607), (16:0/22:6, m/z 864.576) and (18:1/20:4 m/z 866.588). PC hydroperoxy-species are presented as pmol/nmol of the parental non-oxidized PC species. Data is mean ± s.d. of n = 7 pooled animals from two independent experiments, ∗p > 0.05 versus wildtype. (b) MS2 fragmentation patterns of non-oxidized PE with m/z 766.539 (upper panel) and its oxygenated species with m/z 798.538 (lower panel). (c) MS2 fragmentation patterns of non-oxidized CL (upper panel) and its oxygenated species (lower panel). (d) Mass spectra of oxygenated CL before (left upper panel) and after reduction with TPP fragmentation patterns of non-oxidized CL (left upper panel) and representative structures of hydroperoxy- (right upper panel) and hydroxy- (right lower panel) species.
Supplementary Figure 7 Specificity of Liproxstatin-1 against ferroptotic cell death.
For all the inhibitor assays, the cells were pre-incubated with Liproxstatin-1 (500 nM) for 24 h. Then, each lethal compound was added at increasing concentrations for another 48 h, and cell viability was assessed using AquaBluerTM. IC50 depicted in the figures represent the lethal compound LD50 in the presence or absence of Liproxstatin-1. Data shown represents the mean ± s.d. of n = 4 wells of a 96-well plate from an experiment performed one time (nd = not determined).
Supplementary Figure 8 Gpx4 inactivation induces ferroptosis in human kidney cells.
(a) Specificity of active (1S,3R)-RSL3 and inactive (1R,3R)-RSL3 on cell death induction in Pfa1 cells and Pfa1 cells overexpressing Gpx4. Cells were treated with increasing concentrations of both isomers (0-0.5 μM), and cell viability was assessed 24 h thereafter. (b) Impact of active and inactive forms of RSL3 on the viability of primary (HRPEitC) and transformed (HK-2) human tubular epithelial kidney cells. Cells were treated with increasing concentrations of the two isomers (0-0.5 μM), and cell viability was assessed 48 h thereafter. (c) Gpx4 knockdown induces cell death of human kidney tubular cells (HK-2). Impaired viability due to Gpx4 knockdown is rescued by αToc (10 μM) in HK-2 cells (scb, scrambled). (d) Kidney tubular cells (lanes 3, 4) show remarkably high expression levels of Gpx4 that are even higher than in Gpx4 overexpressing Pfa1 cells (lane 1). Gpx4 in overexpressing cells (lane 2) is visualized at approximately 24 kDa due to the presence of an N-terminal tandem affinity purification enhanced (TAPe) tag. (e) siRNA-mediated downregulation of Gpx4 sensitizes to (1S,3R)-RSL3 but not (1R,3R)-RSL3 treatment. Cells were transfected with 20 nmol of siRNA targeting Gpx4 and treated with the compounds 24 h after transfection. Cell viability was determined 24 h after treatment with the chemicals. Data shown in the individual sub-panels represents the mean ± s.d. of n = 3 wells (a, b and e) or n = 4 wells (c) of a 96- (a, b and e) or 6-well (c) plate from a representative experiment performed independently at least two times, ANOVA (∗p < 0.05).
Supplementary Figure 9
Scans of uncropped blots.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2416 kb)
Rights and permissions
About this article
Cite this article
Friedmann Angeli, J., Schneider, M., Proneth, B. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16, 1180–1191 (2014). https://doi.org/10.1038/ncb3064
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3064
This article is cited by
-
Mitochondria-derived methylmalonic acid aggravates ischemia–reperfusion injury by activating reactive oxygen species-dependent ferroptosis
Cell Communication and Signaling (2024)
-
Mechanisms controlling cellular and systemic iron homeostasis
Nature Reviews Molecular Cell Biology (2024)
-
Chains of death
Nature Chemical Biology (2024)
-
CGI1746 targets σ1R to modulate ferroptosis through mitochondria-associated membranes
Nature Chemical Biology (2024)
-
M7G modification of FTH1 and pri-miR-26a regulates ferroptosis and chemotherapy resistance in osteosarcoma
Oncogene (2024)