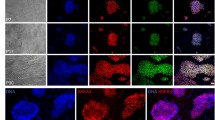
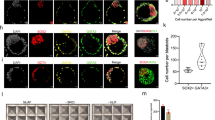
Abstract
Undifferentiated human embryonic stem cells (hESCs) are currently propagated on a relatively small scale as monolayer colonies1,2,3,4,5,6,7. Culture of hESCs as floating aggregates is widely used for induction of differentiation into embryoid bodies8. Here we show that hESC lines can be derived from floating inner cell masses in suspension culture conditions that do not involve feeder cells or microcarriers. This culture system supports prolonged propagation of the pluripotent stem cells as floating clusters without their differentiation into embryoid bodies. HESCs cultivated as aggregates in suspension maintain the expression of pluripotency markers and can differentiate into progeny of the three germ layers both in vitro and in vivo. We further show the controlled differentiation of hESC clusters in suspension into neural spheres. These results pave the way for large-scale expansion and controlled differentiation of hESCs in suspension, which would be valuable in basic and applied research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Reubinoff, B.E., Pera, M.F., Fong, C.Y., Trounson, A. & Bongso, A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 18, 399–404 (2000).
Thomson, J.A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Ludwig, T.E. et al. Derivation of human embryonic stem cells in defined conditions. Nat. Biotechnol. 24, 185–187 (2006).
Richards, M., Fong, C.Y., Chan, W.K., Wong, P.C. & Bongso, A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat. Biotechnol. 20, 933–936 (2002).
Xu, C. et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 19, 971–974 (2001).
Braam, S.R. et al. Feeder-free culture of human embryonic stem cells in conditioned medium for efficient genetic modification. Nat. Protoc. 3, 1435–1443 (2008).
Wang, L. et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood 110, 4111–4119 (2007).
Kurosawa, H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J. Biosci. Bioeng. 103, 389–398 (2007).
McDevitt, T.C. & Palecek, S.P. Innovation in the culture and derivation of pluripotent human stem cells. Curr. Opin. Biotechnol. 19, 527–533 (2008).
Lock, L.T. & Tzanakakis, E.S. Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue Eng. Part A 15, 2051–2063 (2009).
Nie, Y., Bergendahl, V., Hei, D.J., Jones, J.M. & Palecek, S.P. Scalable culture and cryopreservation of human embryonic stem cells on microcarriers. Biotechnol. Prog. 25, 20–31 (2009).
Oh, S.K. et al. Long-term microcarrier suspension cultures of human embryonic stem cells. Stem Cell Res. (Amst.) 4, 4 (2009).
Krawetz, R. et al. Large-scale expansion of pluripotent human embryonic stem cells in stirred suspension bioreactors. Tissue Eng. Part C Methods 8, 8 (2009).
Itsykson, P. et al. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Mol. Cell. Neurosci. 30, 24–36 (2005).
Hoover, C.S. & Martin, R.L. Antibody production and growth of mouse hybridoma cells in Nutridoma media supplements. Biotechniques 8, 76–82 (1990).
Stockinger, H. Serum-free medium for mammalian cells. US patent 5,063,157 (1991).
Amit, M., Shariki, C., Margulets, V. & Itskovitz-Eldor, J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol. Reprod. 70, 837–845 (2004).
Xu, C. et al. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells 23, 315–323 (2005).
Pyle, A.D., Lock, L.F. & Donovan, P.J. Neurotrophins mediate human embryonic stem cell survival. Nat. Biotechnol. 24, 344–350 (2006).
Beattie, G.M. et al. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells 23, 489–495 (2005).
Furue, M.K. et al. Heparin promotes the growth of human embryonic stem cells in a defined serum-free medium. Proc. Natl. Acad. Sci. USA 105, 13409–13414 (2008).
Turetsky, T. et al. Laser-assisted derivation of human embryonic stem cell lines from IVF embryos after preimplantation genetic diagnosis. Hum. Reprod. 23, 46–53 (2008).
Herszfeld, D. et al. CD30 is a survival factor and a biomarker for transformed human pluripotent stem cells. Nat. Biotechnol. 24, 351–357 (2006).
Baker, D.E. et al. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat. Biotechnol. 25, 207–215 (2007).
Mitalipova, M.M. et al. Preserving the genetic integrity of human embryonic stem cells. Nat. Biotechnol. 23, 19–20 (2005).
Lyons, A.B. & Parish, C.R. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171, 131–137 (1994).
Watanabe, K. et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25, 681–686 (2007).
Lee, S.H., Lumelsky, N., Studer, L., Auerbach, J.M. & McKay, R.D. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat. Biotechnol. 18, 675–679 (2000).
Yan, Y. et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells 23, 781–790 (2005).
Gropp, M. & Reubinoff, B. Lentiviral vector-mediated gene delivery into human embryonic stem cells. Methods Enzymol. 420, 64–81 (2006).
Acknowledgements
We are grateful to the following members of the Hadassah Human Embryonic Stem Cell Research Center: M. Gropp, M. Aharonowiz and O. Singer for technical assistance; S. Tennenbaum for editing the manuscript. We thank K.M. Yamada (National Institute of Dental and Craniofacial Research, National Institutes of Health) for providing anti-fibronectin antibody, N. Benvenisty for the QPCR primers and WiCell Research Institute for providing H7 hESCs. This research was supported by a gift from Judy and Sidney Swartz, the Sidney Swartz Chair in Human Embryonic Stem Cell Research and Legacy Heritage Fund.
Author information
Authors and Affiliations
Contributions
D.S. designed and performed the experiments, analyzed the data and wrote the manuscript; H.K. and M.C. performed the neural differentiation study; S.E.-R. conducted immunostainings and confocal analysis; Y.G. performed the teratoma studies; P.I. contributed to developing the concept of suspension culture; T.T. contributed to the experiments; M.I. performed PCR analysis; E.A. contributed to embryo recruitment, culture and isolation of inner cell masses; R.R. and Y.B.-Z. conducted karyotype analysis. B.R. conceived the study and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
B.R. is the CSO and holds shares in CellCure Neurosciences Ltd. However, the project was not funded by CellCure Neurosciences Ltd. and the company has no rights in its results.
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–10 (PDF 795 kb)
Rights and permissions
About this article
Cite this article
Steiner, D., Khaner, H., Cohen, M. et al. Derivation, propagation and controlled differentiation of human embryonic stem cells in suspension. Nat Biotechnol 28, 361–364 (2010). https://doi.org/10.1038/nbt.1616
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1616
This article is cited by
-
Cell fiber-based three-dimensional culture system for highly efficient expansion of human induced pluripotent stem cells
Scientific Reports (2017)
-
Techniques of Human Embryonic Stem Cell and Induced Pluripotent Stem Cell Derivation
Archivum Immunologiae et Therapiae Experimentalis (2016)
-
Optimization of agitation speed in spinner flask for microcarrier structural integrity and expansion of induced pluripotent stem cells
Cytotechnology (2016)
-
Human embryonic stem cell cultivation: historical perspective and evolution of xeno-free culture systems
Reproductive Biology and Endocrinology (2015)