Abstract
Although many studies indicate the interplay of genetic and environmental factors in the etiology of autism spectrum disorder (ASD), our limited understanding of the underlying mechanisms hampers the development of effective ways of detecting and preventing the disorder. Recent studies support the hypothesis that prenatal androgen exposure contributes to the development of ASD. This would suggest that maternal polycystic ovary syndrome (PCOS), a condition associated with excess androgens, would increase the risk of ASD in the offspring. We conducted a matched case–control study nested within the total population of Sweden (children aged 4–17 who were born in Sweden from 1984 to 2007). The sample consisted of 23 748 ASD cases and 208 796 controls, matched by birth month and year, sex and region of birth. PCOS and ASD were defined from ICD codes through linkage to health-care registers. Maternal PCOS increased the odds of ASD in the offspring by 59%, after adjustment for confounders (odds ratio (OR) 1.59, 95% confidence interval (CI) 1.34–1.88). The odds of offspring ASD were further increased among mothers with both PCOS and obesity, a condition common to PCOS that is related to more severe hyperandrogenemia (OR 2.13, 95% CI 1.46–3.10). Risk estimates did not differ between sexes. In conclusion, children of women with PCOS appear to have a higher risk of developing ASD. This finding awaits confirmation, and exploration of potentially underlying mechanisms, including the role of sex steroids in the etiology of ASD.
Similar content being viewed by others
Introduction
Autism spectrum disorders (ASDs) are more common in boys, with male-to-female ratios reported from 3:1 up to 16:1.1, 2 Although the sex bias may result from difficulties in diagnosing autism in girls,3, 4 it also suggests that factors in male versus female sexual development may be important for ASD.5 Neuronal circuits regulating behavioral domains relevant to ASD, such as communication and social interaction,6 are influenced by sex hormones.7 Children with ASD share more morphological features of male brains, such as larger brain volume overall and larger amygdalas,8, 9 exhibit testosterone-related hemispheric asymmetries10 and have more male-typical cognitive features.11, 12 Genes regulating sex hormones have also been associated with ASD.13 Lastly, elevated androgens have been noted after diagnosis with ASD, especially in females.14, 15
One hypothesis integrating these lines of evidence is that exposure to androgens early in life can modify brain development and mediate the risk of ASD in both genders.16 A recent study examining steroids in amniotic fluid found that children who later developed ASD had higher levels of hormones along the Δ4 steroidogenic pathway, including the androgens testosterone and androstenedione.17 However, the source of steroids could not be identified, and the fetus, mother, placenta or other environmental factors might all have contributed to the observed differences between cases and controls.
Polycystic ovary syndrome (PCOS) is a common endocrine disorder, affecting ~5–15% of women of fertile age and characterized by hyperandrogenism, ovarian dysfunction and polycystic ovarian morphology.18, 19, 20 A small clinical study examined scores on autism-rating scales in the offspring of women with PCOS and found an association with higher scores among daughters of affected mothers.21 Two other studies have explored maternal PCOS diagnosis in the context of maternal infertility as a risk factor for ASD, although these studies were small and lacked statistical power.22, 23 There have been no large, population-based studies on maternal PCOS and the risk of clinically overt ASD.
Here we examine the relationship between maternal diagnosis of PCOS and the risk of ASD in the offspring in a nationwide case–control study based on prospectively collected health register data.
Materials and Methods
Study population
All data are derived from linkages to national registers held by Statistics Sweden and the National Board of Health and Welfare. The registers retain routinely collected health and sociodemographic data on the entire population of Sweden. The registers are crosslinked via each person’s unique national registration number assigned to all residents at birth or upon migration to Sweden.24 The eligible study population consisted of all individuals born in Sweden from 1984 to 2011 and followed until 31 December 2011. We matched all cases of ASD in the study population with up to 10 living controls who were without a diagnosis of ASD at the end of follow-up period by birth month and year, sex and region of birth. This study was approved by the regional ethical review board at Karolinska Institutet. Informed consent was not required for the analysis of anonymized register data.
ASD case ascertainment
The ascertainment of ASD is based on data from the National Patient Register (NPR) that contains data on all inpatient care in Sweden since 1973 and outpatient specialist physician care since 2001. ASD case status as of 31 December 2011, was defined as a recorded diagnosis of ICD-9: 299 or ICD-10: F84. A validation study using a CDC protocol for medical record review of autism diagnoses confirmed the presence of DSM-IV autism in 83 out of 88 persons (94.3%) with a diagnosis recorded in the NPR.25 To assure that children were old enough to receive an accurate diagnosis,4 children born after 2007 were excluded.
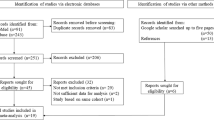
After exclusion of children not born in Sweden, born after 2007, from a multiple birth, adopted and those with missing covariate data (see Figure 1), the final sample consisted of 23 748 ASD cases matched to 208 796 controls, with case–control ratio varying from 1:3 to 1:10.
Derivation of the Swedish Youth Cohort analytical sample.
Information on presence or absence of comorbid intellectual disability was available for a subsample of the total Swedish population, the Stockholm Youth Cohort (Supplementary Information).3, 4
Exposure
Maternal PCOS case status was classified according to any lifetime recorded diagnosis (ICD-8: 256.90, ICD-9: 256E and ICD-10: E28.2) within the NPR, supplemented by diagnoses in the Medical Birth Register (MBR). The coverage of the NPR is ~99% of all somatic discharge diagnoses, and the validity of the diagnoses is generally high.26 The MBR includes information on ~98% of births in Sweden, with information on the mother, pregnancy, delivery and neonatal period.
We used diagnoses of PCOS diagnosed at any time in life as the exposure, since PCOS is a longitudinal disorder with hormonal and metabolic manifestations, such as increased androgens, throughout the lifespan.27, 28 Thus, the disorder was considered to be present at pregnancy regardless of timing of the recorded diagnosis.
Covariates
Covariates were identified a priori as potential confounding factors and/or risk factors for ASD. Maternal and paternal ages at the time of birth were categorized as <25, 25–29, 30–34, 35–39, ⩾40 years.29 Data on family income after deduction of taxes were obtained from the Integrated Database for Labor Market Research, adjusted for family size, and categorized into quintiles according to the birth year to account for inflation.30 The highest education of either parent at the time of birth was classified as compulsory (⩽9 years), high school (10–12 years) or university or higher (⩾13 years).30 Parental history of psychiatric inpatient and outpatient treatment before the birth of the index child (yes/no) was defined as any psychiatric diagnosis (Chapter V of ICD-8 and -9 or Chapter F of ICD-10) recorded in the NPR. Maternal migrant status was categorized as born in Sweden or outside.
We explored whether obstetric complications influenced the findings of our main analysis, because of reports of increased obstetric complications in mothers with PCOS31 and associations of obstetric complications with ASD.32, 33 Apgar score at 5 min was categorized as <7 or⩾7 and supplemented by Apgar score at 1 min when the data on the score at 5 min were not available. Size for gestational age was categorized as normal, small for gestational age or large for gestational age. Gestational age was categorized as preterm (<37 weeks), term (37–41 weeks) and post term (⩾ 42 weeks). Gestational diabetes was classified according to ICD-9: 648 W and ICD-10: O24.4 diagnoses recorded within the MBR, or in the NPR during the 9 months before and 1 month after birth of the index child. A similar strategy was used for identifying pre-eclampsia using the following codes: ICD-8: 637.03–637.04, 637.09–637.10, ICD-9: 642E-642H and ICD-10: O14-O15. Birth order was categorized as first born or not according to the MBR.
PCOS increases infertility and women with PCOS more often use assisted reproductive technology.19 Information on assisted reproductive technology, including in vitro fertilization and intra-cytoplasmic injection, was available in the MBR from 1995 onwards.
Obesity and other features of the metabolic syndrome, including insulin resistance, hypertension and dyslipidemia, are common in PCOS and are related to more severe hyperandrogenemia in women with PCOS.34, 35, 36 Furthermore, elevated pre-pregnancy body mass index (BMI) has been associated with the risk of ASD.37 Thus, we explored the roles of pre-pregnancy BMI and metabolic syndrome in the relationship between maternal PCOS and offspring ASD.
To calculate BMI (kg m−2) we used weight and height data recorded by midwives at the first visit to a maternal health clinic, at median 10.6 (interquartile range: 9.0–12.6) gestational weeks. Weights <40 kg or >140 kg were censored, as were heights <140 cm and >200 cm. These end points were not captured during 1990–1991 and were incomplete in other years; in total, data were available for 70% of the mother–child pairs in our study. Maternal baseline BMI was categorized by standard convention:38 underweight (BMI<18.5), normal (18.5⩽BMI<25), overweight (25⩽BMI<30) and obese (BMI⩾30). Mothers were classified as having a prior diagnosis of diabetes mellitus according to the codes ICD-8: 250, ICD-9: 250, 648 A, 790C and ICD-10: E10-E11, O24.0-O24.1, if at least one diagnosis was recorded before and including the birth year of the index child (in the NPR and MBR). Diabetes was not differentiated as type I or type II, as these distinctions were not recorded before the use of ICD-10 coding. A similar strategy was used to identify essential hypertension, using the following ICD-codes, ICD-8: 400–404, ICD-9: 401–404, 642 A-642C and ICD-10: I10-I13, O10-O11.
Statistical analysis
Analyses were performed using SPSS, version 20 (IBM, Armonk, NY, USA) and using R version 3.1.2. Conditional logistic regression was used to evaluate the association between maternal diagnosis with PCOS and offspring ASD, first as a crude (unadjusted) estimate (Model 1) and then in a model adjusted for maternal/paternal age, parental psychiatric history, parental income/education and maternal country of birth (Model 2). Mothers without PCOS were used as the reference group. In order to explore whether accounting for obstetric complications influenced the estimated odds ratios (ORs) from Model 2, we used a model additionally adjusted for the following obstetric factors: Apgar score, gestational age, size for gestational age, maternal gestational diabetes and pre-eclampsia (Model 3). We also examined results in sex-stratified models.
The roles of obesity and the metabolic syndrome in the PCOS and offspring ASD relationship were also explored in the subset of mother–child pairs with complete BMI data (Figure 1).
First, we repeated the analysis of maternal PCOS and ASD (Models 1–3) in the sub-cohort with BMI data. We tested for potential confounding effects of BMI and features of the metabolic syndrome by further adjusting for BMI, in models that excluded (Model 4) or included (Model 5) obstetric complications. Model 5 was further adjusted to include additional features of the metabolic syndrome; a diagnosis of essential hypertension and/or diabetes (Model 6). Model 5 was also adjusted for birth order (Model 7).
Lastly, we tested for the effects of worsening cardiometabolic profiles amongst women with PCOS, because of the associations of such profiles with hyperandrogenemia in PCOS. We explored whether the risk of offspring ASD was different amongst mothers with the following a priori defined mutually exclusive categories: no PCOS, PCOS, PCOS with obesity and PCOS with obesity and indications of metabolic syndrome. Mothers without PCOS were used as the reference group. Women without PCOS but with obesity, diabetes or essential hypertension were included in the reference group to avoid creation of a non-representative control group. We formally tested for interaction between PCOS and obesity by examining a model that included the cross-product term.
Secondary analyses
For the Stockholm Youth Cohort case–control subsample, the outcome was stratified as ASD with and without intellectual disability and analyses (Models 1–3) were repeated.
Sensitivity analyses
PCOS increases the risk of infertility and infertility treatments might influence offspring health; therefore, we repeated analysis of maternal PCOS for children born after 1995, adjusting for use of assisted reproductive technology.
Results
A higher proportion of mothers of ASD cases had a diagnosis of PCOS compared with mothers of non-cases (Table 1). ASD cases were more likely to have an older father, a mother born outside of Sweden, parental history of psychiatric care before delivery, lower household income, lower parental education and obstetric complications (Table 1).
Maternal diagnosis of PCOS was associated with higher odds of ASD in both unadjusted and adjusted models (Table 2). Neither adjustment for obstetric complications (Model 3, Table 2) nor adjustment for use of assisted reproductive technology (sensitivity analyses, data not shown) substantially attenuate the OR for ASD. In sex-stratified analyses, ORs were similar for both boys and girls (Table 3). In a secondary analysis, we found that associations of maternal PCOS with ASD with or without intellectual disability did not meaningfully differ, as indications of elevated risk were found with both outcomes (see Supplementary Information).
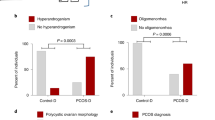
More women with PCOS had elevated BMI, as compared with women without PCOS. Among mothers with PCOS, 1.3% were underweight, 41.2% normal weight, 31.7% overweight and 25.7% obese. Corresponding proportions among mothers without PCOS were 3.8, 67.7, 20.9 and 7.6%, respectively.
Maternal diagnosis of PCOS was associated with higher odds of ASD in the subset of women with BMI data and ORs in unadjusted and adjusted models were similar compared with the whole study population (Table 2). The ORs were attenuated but remained significant after adjustment for BMI (Models 4 and 5). Adjusting Model 5 further for hypertonia and diabetes or for birth order did not substantially attenuate the ORs for ASD (results not shown).
In stratified analysis, ORs were higher for mothers with PCOS and obesity compared with mothers with PCOS only (Table 4). Power to examine mothers with PCOS and indications of metabolic syndrome as a separate group was limited by the small number of affected mothers, although statistical testing indicated a trend of increasing odds with increasing PCOS severity category. Likelihood-ratio testing for interaction between PCOS and obesity found no statistical evidence of an interaction in terms of risk for offspring ASD (results not shown).
Discussion
To the best of our knowledge, this is the first population-based study with adequate power to examine the relationship between maternal diagnosis of PCOS and the risk of ASD in the offspring. We found that maternal diagnosis of PCOS increased the risk of ASD, even after adjusting for potential confounders. Obesity among women with PCOS appeared to further increase the risk of ASD in the offspring. The risk associated with PCOS was similar in males and females.
A previous study by Palomba et al.21 followed 30 pregnant women with PCOS and 45 healthy controls and scored their offspring on autism-rating scales. Although none of these women had a child with ASD, the study found higher autism-rating scores in daughters of mothers with PCOS, whereas a nonsignificant trend was observed among their sons. Our study adds evidence of an association between maternal PCOS and the risk of clinically diagnosed ASD in the offspring, although we found that the association between maternal PCOS and ASD did not differ between sexes. Women with PCOS experience increased risk of infertility and a few prior studies have explored the relationship between maternal infertility and ASD. One study found no association between maternal infertility diagnoses, including PCOS, and maternally reported offspring ASD.23 Similarly in another study, no association was found between maternal hormonal infertility and offspring ASD.22 However, neither of these studies had adequate numbers of mothers with PCOS and the latter grouped PCOS with other maternal hormonal conditions.
Interestingly, two previous studies have examined the hypothesis of comorbidity between PCOS and autism. Ingudomnukul et al.39 found PCOS to be more commonly reported by women with ASD but not by mothers of children with ASD, although lack of statistical power could potentially explain this equivocal finding. The finding of a higher prevalence of PCOS among women with a diagnosis of ASD was not replicated in a recent study by Pohl et al.,40 although the authors attributed this to a possible underdiagnosis of PCOS in ASD women. However, Pohl et al. found that other androgen-related medical conditions were more common in females with ASD, including precocious puberty, early growth spurt and reproductive dysfunction, similar to reproductive and developmental disturbances that have been reported in daughters of women with PCOS.41
These studies support the hypothesis that elevated prenatal androgens, such as testosterone, may contribute to ASD in both sexes.16 Prenatal androgens may contribute to the development of autism as they shape neurological development through lasting organizational effects on pre- and postnatal brain development and/or fetal epigenetic programming.5 For example, sex steroids may affect neuronal density and dendritic morphology42 that are thought to be atypical in autism.43, 44 Sex steroids also exert epigenetic influences that may result in altered neuronal development and aberrant synapse function and morphology.45, 46
Most women with PCOS have clinical hyperandrogenemia, which is aggravated by obesity.34, 35, 47, 48, 49, 50, 51 PCOS could, therefore, provide a potential source of androgen excess for the fetus, although this is less clear. Circulating levels of androgens are increased during gestation in women with PCOS52 and there are reports that newborns of women with PCOS have atypical levels of sex steroids.53, 54, 55 Barry et al.54 demonstrated higher testosterone levels in cord blood of newborn females of mothers with PCOS, but Maliqueo et al.55 did not replicate this finding. Maliqueo et al. found that placentas of women with PCOS show abnormal steroidogenic activity and increased capacity for producing androgens that could potentially contribute to fetal androgen exposure. This is supported by evidence that daughters of mothers with PCOS show alterations in ovarian and metabolic function that are related to prenatal androgen programming.41, 56, 57 Women with PCOS exhibit insulin resistance and increased insulin resistance during gestation.52, 58 Gestational hyperinsulinemia is associated with abnormal placental steroidogenesis,59 hyperandrogenemia60 and increased testosterone in amniotic fluid.61 Further investigation is required to determine how the hormonal and metabolic disturbances in women with PCOS might affect fetal neurodevelopment.
Baron-Cohen et al.17 examined amniotic fluid samples from children with a clinical diagnosis of ASD and healthy controls and found elevations among ASD cases in levels of all hormones along the Δ4 sex-steroid biosynthetic pathway, including progesterone, 17a-hydroxy-progesterone, androstenedione, testosterone and cortisol. The authors concluded that there is a broadly elevated fetal steroidogenic activity in autism rather than the abnormality being restricted to a specific hormone. Dysregulation of the Δ4 as well as the Δ5 steroidogenic pathways has also been reported in PCOS and studies among women with PCOS have found abnormalities in levels of the cytochrome P450-containing enzymes that mediate the conversion of hormones along these pathways.62, 63, 64 Dysregulated steroidogenic activity and elevated androgens might thus explain an association between maternal PCOS and offspring autism.
As an alternative and potentially complementary explanation, the association between maternal PCOS and the risk of ASD could be explained by genetic influences related to sex steroids that are common for PCOS and autism. For example, the gene encoding the cytochrome P450-containing enzyme CYP17 that catalyzes conversions of hormones along the Δ4 pathway has been associated with both autism65 and PCOS,66 although the evidence is inconclusive.13, 67
Elevated BMI is a clinical feature of PCOS and obesity was common among mothers with PCOS in our study. In women with PCOS, obesity is closely related to hyperinsulinemia that may increase P450-enzymatic activity and lead to hyperandrogenemia.68, 69 Hyperandrogenism contributes to adiposity in PCOS and obesity in turn exacerbates hormonal and metabolic disturbances of PCOS.34 Studies have demonstrated that obese women with PCOS have decreased sex hormone-binding globulin, increased total testosterone and increased free androgen index, as compared with their normal weight counterparts.34, 49, 50 Hyperandrogenism in women with PCOS is also associated with worse cardiometabolic profile.34, 35, 36 Our finding that obesity among mothers with PCOS further increased the risk of offspring ASD provides some evidence that the maternal metabolic and hormonal environment in PCOS, rather than shared genetic influences, is likely to contribute to the risk of ASD. There was a trend in risk for ASD with worsening cardiometabolic profiles of mothers with PCOS in our study, although this analysis was based on small numbers of women with PCOS, obesity and other features of the metabolic syndrome.
Elevated pre-pregnancy BMI has been independently associated with the risk of offspring ASD in population-level studies.37, 70 However, a recent Swedish study indicated that BMI might be a proxy marker for other familial risk factors, since the relationship between maternal elevated BMI and ASD risk was not apparent in sibling analyses.37 Adjusting for BMI attenuated, but did not fully explain, the relationship between PCOS and the risk of ASD in our study. Given the bidirectional relationship between adiposity and hyperandrogenism in PCOS and non-PCOS populations,71, 72 the results of adjusting for BMI should be interpreted with caution because of the risk of overadjustment. It might also be that genetic factors or other familial influences shared between PCOS and adiposity are related to the risk of ASD in the offspring.
Strengths of the study include the large, population-based sample, validated ASD case ascertainment3, 25 and adjustment for a range of possible confounders. Our study has limitations. Women with PCOS not seeking medical assistance for symptoms of the syndrome would be misclassified as not having the disorder. The prevalence of PCOS that we found was lower than the estimated population prevalence for PCOS. Although register-based diagnoses are objective and not subject to recall bias, we might have captured women with more severe PCOS as exposed and the findings may not be generalizable to all women with PCOS. Fertility treatments are common among women with PCOS and we were able to adjust for use of in vitro fertilization and intra-cytoplasmic injection. However, we did not use information on ovulation induction therapies that have been associated with ASD in previous studies.23, 73 Thus, mediation by use of ovulation-inducing drugs cannot be ruled out. PCOS accounts for ~80% of clinical hypernadrogenemia in general population.74 Although most patients with PCOS have clinical hyperandrogenemia, non-hyperandrogenic PCOS cases also exist. Misclassification of women with hyperandrogenemia as non-exposed and of non-hyperandrogenic women with PCOS as exposed might lead to an underestimation of the association, if hyperandrogenemia is, as hypothesized, the biological link between PCOS and ASD. Nevertheless, we found that a diagnosis of PCOS increases the risk of clinically overt ASD in the offspring. Data on BMI were available only for a subsample of the study population. However, we found few differences between women with and without baseline BMI data in characteristics such as socioeconomic factors, suggesting that the BMI data are missing at random.37 Finally, it was not possible to characterize risks amongst offspring of women with PCOS and other aspects of the metabolic syndrome, due to the limited number of women with these diagnoses.
In conclusion, the present study provides evidence that children of mothers with PCOS have an increased risk of developing ASD, regardless of sex. Our results support that early life androgen exposure may be important for the development of autism in both sexes and call for further research on the mechanisms involving sex steroids in the etiology of ASD.
References
Gillberg C, Cederlund M, Lamberg K, Zeijlon L . Brief report: "The autism epidemic". The registered prevalence of autism in a swedish urban area. J Autism Dev Disord 2006; 36: 429–435.
Rutter M, Caspi A, Moffitt TE . Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. J Child Psychol Psychiatry 2003; 44: 1092–1115.
Idring S, Rai D, Dal H, Dalman C, Sturm H, Zander E et al. Autism spectrum disorders in the Stockholm Youth Cohort: design, prevalence and validity. PloS One 2012; 7: e41280.
Idring S, Lundberg M, Sturm H, Dalman C, Gumpert C, Rai D et al. Changes in prevalence of autism spectrum disorders in 2001-2011: findings from the Stockholm Youth Cohort. J Autism Dev Disord 2014; 45: 1766–1773.
Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, Knickmeyer R . Why are autism spectrum conditions more prevalent in males? Plos Biol 2011; 9: e1001081.
Happe F, Ronald A . The 'fractionable autism triad': a review of evidence from behavioural, genetic, cognitive and neural research. Neuropsychol Rev 2008; 18: 287–304.
Auyeung B, Lombardo MV, Baron-Cohen S . Prenatal and postnatal hormone effects on the human brain and cognition. Pflugers Arch 2013; 465: 557–571.
Courchesne E, Campbell K, Solso S . Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res 2011; 1380: 138–145.
Nordahl CW, Scholz R, Yang XW, Buonocore MH, Simon T, Rogers S et al. Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders. A longitudinal study. Arch Gen Psychiatry 2012; 69: 53–61.
Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Kennedy DN, Filipek PA et al. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain 2005; 128: 213–226.
Baron-Cohen S . The extreme male brain theory of autism. Trends Cogn Sci 2002; 6: 248–254.
Jolliffe T, BaronCohen S . Are people with autism and Asperger syndrome faster than normal on the embedded figures test? J Child Psychol Psychiatry 1997; 38: 527–534.
Zettergren A, Jonsson L, Johansson D, Melke J, Lundstrom S, Anckarsater H et al. Associations between polymorphisms in sex steroid related genes and autistic-like traits. Psychoneuroendocrinology 2013; 38: 2575–2584.
Bejerot S, Eriksson JM, Bonde S, Canstrom K, Humble MB, Eriksson E . The extreme male brain revisited: gender coherence in adults with autism spectrum disorder. Br J Psychiatry 2012; 201: 116–123.
Ruta L, Ingudomnukul E, Taylor K, Chakrabarti B, Baron-Cohen S . Increased serum androstenedione in adults with autism spectrum conditions. Psychoneuroendocrinology 2011; 36: 1154–1163.
Baron-Cohen S, Knickmeyer RC, Belmonte MK . Sex differences in the brain: Implications for explaining autism. Science 2005; 310: 819–823.
Baron-Cohen S, Auyeung B, Norgaard-Pedersen B, Hougaard D, Abdallah M, Melgaard L et al. Elevated fetal steroidogenic activity in autism. Mol Psychiatry 2015; 20: 369–376.
Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF . A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab 2000; 85: 2434–2438.
Ehrmann DA . Medical progress: polycystic ovary syndrome. N Engl J Med 2005; 352: 1223–1236.
Sanchon R, Gambineri A, Alpanes M, Angeles Martinez-Garcia M, Pasquali R, Escobar-Morreale HF . Prevalence of functional disorders of androgen excess in unselected premenopausal women: a study in blood donors. Hum Reprod 2012; 27: 1209–1216.
Palomba S, Marotta R, Di Cello A, Russo T, Falbo A, Orio F et al. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study. Clin Endocrinol 2012; 77: 898–904.
Lyall K, Baker A, Hertz-Picciotto I, Walker CK . Infertility and its treatments in association with autism spectrum disorders: a review and results from the CHARGE study. Int J Environ Res Public Health 2013; 10: 3715–3734.
Lyall K, Pauls DL, Spiegelman D, Santangelo SL, Ascherio A . Fertility therapies, infertility and autism spectrum disorders in the Nurses' Health Study II. Paediatr Perinat Epidemiol 2012; 26: 361–372.
Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A . The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009; 24: 659–667.
Ludvigsson JF, Reichenberg A, Hultman CM, Murray JA . A nationwide study of the association between celiac disease and the risk of autistic spectrum disorders. JAMA Psychiatry 2013; 70: 1224–1230.
Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450.
Goverde AJ, Westerveld HE, Verhulst SM, Fauser BC . Polycystic ovary syndrome as a developmental disorder. Expert Rev Obstet Gynecol 2008; 3: 775–787.
Pinola P, Piltonen TT, Puurunen J, Vanky E, Sundstrom-Poromaa I, Stener-Victorin E et al. Androgen profile through life in women with polycystic ovary syndrome: a Nordic multicenter collaboration study. J Clin Endocrinol Metab 2015; 100: 3400–3407.
Idring S, Magnusson C, Lundberg M, Ek M, Rai D, Svensson AC et al. Parental age and the risk of autism spectrum disorders: findings from a Swedish population-based cohort. Int J Epidemiol 2014; 43: 107–115.
Rai D, Golding J, Magnusson C, Steer C, Lewis G, Dalman C . Prenatal and early life exposure to stressful life events and risk of autism spectrum disorders: population-based studies in Sweden and England. PloS One 2012; 7: e38893.
Roos N, Kieler H, Sahlin L, Ekman-Ordeberg G, Falconer H, Stephansson O . Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study. BMJ 2011; 343: d6309.
Abel KM, Dalman C, Svensson AC, Susser E, Dal H, Idring S et al. Deviance in fetal growth and risk of autism spectrum disorder. Am J Psychiatry 2013; 170: 391–398.
Gardener H, Spiegelman D, Buka SL . Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics 2011; 128: 344–355.
Daan NMP, Louwers YV, Koster MPH, Eijkemans MJC, de Rijke YB, Lentjes EWG et al. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: who is really at risk? Fertil Steril 2014; 102: 1444–1451.
Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC . The insulin-related ovarian regulatory system in health and disease. Endocrine Rev 1999; 20: 535–582.
Albu A, Radian S, Fica S, Barbu C . Biochemical hyperandrogenism is associated with metabolic syndrome independently of adiposity and insulin resistance in Romanian polycystic ovary syndrome patients. Endocrine 2015; 48: 696–704.
Gardner RM, Lee BK, Magnusson C, Rai D, Frisell T, Karlsson H et al. Maternal body mass index during early pregnancy, gestational weight gain, and risk of autism spectrum disorders: Results from a Swedish total population and discordant sibling study. Int J Epidemiol 2015; 44: 870–883.
WHO Expert Committee on Physical Status: the Use and Interpretation of Anthropometry Physical status: the use and interpretation of anthropometry. World Health Organization: Geneva, 1995; 452, Technical Report Series no. 854.
Ingudomnukul E, Baron-Cohen S, Wheelwright S, Knickmeyer R . Elevated rates of testosterone-related disorders in women with autism spectrum conditions. Horm Behav 2007; 51: 597–604.
Pohl A, Cassidy S, Auyeung B, Baron-Cohen S . Uncovering steroidopathy in women with autism: a latent class analysis. Mol Autism 2014; 5: 27.
Maliqueo M, Sir-Petermann T, Perez V, Echiburu B, de Guevara AL, Galvez C et al. Adrenal function during childhood and puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2009; 94: 3282–3288.
McCarthy MM, Arnold AP . Reframing sexual differentiation of the brain. Nat Neurosci 2011; 14: 677–683.
Wegiel J, Flory M, Kuchna I, Nowicki K, Ma SY, Imaki H et al. Stereological study of the neuronal number and volume of 38 brain subdivisions of subjects diagnosed with autism reveals significant alterations restricted to the striatum, amygdala and cerebellum. Acta Neuropathol Commun 2014; 2: 141.
Bringas ME, Carvajal-Flores FN, Lopez-Ramirez TA, Atzori M, Flores G . Rearrangement of the dendritic morphology in limbic regions and altered exploratory behavior in a rat model of autism spectrum disorder. Neuroscience 2013; 241: 170–187.
Kigar SL, Auger AP . Epigenetic mechanisms may underlie the aetiology of sex differences in mental health risk and resilience. J Neuroendocrinol 2013; 25: 1141–1150.
Olde Loohuis NF, Kole K, Glennon JC, Karel P, Van der Borg G, Van Gemert Y et al. Elevated microRNA-181c and microRNA-30d levels in the enlarged amygdala of the valproic acid rat model of autism. Neurobiol Dis 2015; 80: 42–53.
Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 2009; 91: 456–488.
Huang A, Brennan K, Azziz R . Prevalence of hyperandrogenemia in the polycystic ovary syndrome diagnosed by the National Institutes of Health 1990 criteria. Fertil Steril 2010; 93: 1938–1941.
Kiddy DS, Sharp PS, White DM, Scanlon MF, Mason HD, Bray CS et al. Differences in clinical and endocrine features between obese and non-obese subjects with polycystic ovary syndrome—an analysis of 263 consecutive cases. Clin Endocrinol 1990; 32: 213–220.
Lim SS, Norman RJ, Davies MJ, Moran LJ . The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev 2013; 14: 95–109.
Livadas S, Pappas C, Karachalios A, Marinakis E, Tolia N, Drakou M et al. Prevalence and impact of hyperandrogenemia in 1,218 women with polycystic ovary syndrome. Endocrine 2014; 47: 631–638.
Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE . Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod 2002; 17: 2573–2579.
Anderson H, Fogel N, Grebe SK, Singh RJ, Taylor RL, Dunaif A . Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. J Clin Endocrinol Metab 2010; 95: 2180–2186.
Barry JA, Kay AR, Navaratnarajah R, Iqbal S, Bamfo J, David AL et al. Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J Obstet Gynaecol 2010; 30: 444–446.
Maliqueo M, Lara HE, Sanchez F, Echiburu B, Crisosto N, Sir-Petermann T . Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2013; 166: 151–155.
Crisosto N, Echiburu B, Maliqueo M, Perez V, de Guevara AL, Preisler J et al. Improvement of hyperandrogenism and hyperinsulinemia during pregnancy in women with polycystic ovary syndrome: possible effect in the ovarian follicular mass of their daughters. Fertil Steril 2012; 97: 218–224.
Sir-Petermann T, Codner E, Perez V, Echiburu B, Maliqueo M, de Guevara AL et al. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2009; 94: 1923–1930.
Sir-Petermann T, Echiburu B, Maliqueo MM, Crisosto N, Sanchez F, Hitschfeld C et al. Serum adiponectin and lipid concentrations in pregnant women with polycystic ovary syndrome. Hum Reprod 2007; 22: 1830–1836.
Uzelac PS, Li X, Lin J, Neese LD, Lin L, Nakajima ST et al. Dysregulation of Leptin and Testosterone Production and Their Receptor Expression in the Human Placenta with Gestational Diabetes Mellitus. Placenta 2010; 31: 581–588.
Uzelac PS, Lee J, Zhang XH, Paulson RJ, Miller DA, Stanczyk FZ . Gestational diabetes augments hyperandrogenemia in the third trimester of pregnancy. J Soc Gynecol Invest 2005; 12: 282A–282A.
Barbieri RL, Saltzman DH, Torday JS, Randall RW, Frigoletto FD, Ryan KJ . Elevated concentrations of the beta-subunit of human chorionic-gonadotropin and testosterone in the amniotic-fluid of gestations of diabetic mothers. Am J Obstet Gynecol 1986; 154: 1039–1043.
de Medeiros SF, Barbosa JS, Yamamoto MMW . Comparison of steroidogenic pathways among normoandrogenic and hyperandrogenic polycystic ovary syndrome patients and normal cycling women. J Obstet Gynaecol Res 2015; 41: 254–263.
Doi SAR, Al-Zaid M, Towers PA, Scott CJ, Al-Shoumer KAS . Steroidogenic alterations and adrenal androgen excess in PCOS. Steroids 2006; 71: 751–759.
Rosenfield RL, Barnes RB, Cara JF, Lucky AW . Dysregulation of cytochrome-P450C17-alpha as the cause of polycystic ovarian syndrome. Fertil Steril 1990; 53: 785–791.
Chakrabarti B, Dudbridge F, Kent L, Wheelwright S, Hill-Cawthorne G, Allison C et al. Genes related to sex steroids, neural growth, and social-emotional behavior are associated with autistic traits, empathy, and asperger syndrome. Autism Res 2009; 2: 157–177.
Pusalkar M, Meherji P, Gokral J, Chinnaraj S, Maitra A . CYP11A1 and CYP17 promoter polymorphisms associate with hyperandrogenemia in polycystic ovary syndrome. Fertil Steril 2009; 92: 653–659.
Li Y, Liu F, Luo S, Hu H, Li XH, Li SW . Polymorphism T -> C of gene CYP17 promoter and polycystic ovary syndrome risk: a meta-analysis. Gene 2012; 495: 16–22.
Ehrmann DA, Rosenfield RL, Barnes RB, Brigell DF, Sheikh Z . Detection of functional ovarian hyperandrogenism in women with androgen excess. N Engl J Med 1992; 327: 157–162.
Nestler JE, Jakubowicz DJ . Lean women with polycystic ovary syndrome respond to insulin reduction with decreases in ovarian P450c17 alpha activity and serum androgens. J Clin Endocrinol Metab 1997; 82: 4075–4079.
Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 2012; 129: e1121–e1128.
Bohler H Jr., Mokshagundam S, Winters SJ . Adipose tissue and reproduction in women. Fertil Steril 2010; 94: 795–825.
McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ et al. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab 2006; 91: 1714–1722.
Bay B, Mortensen EL, Hvidtjorn D, Kesmodel US . Fertility treatment and risk of childhood and adolescent mental disorders: register based cohort study. BMJ 2013; 347: f3978.
Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab 2004; 89: 453–462.
Acknowledgements
We thank Lena Jörgensen for her assistance with the preparation of this manuscript. This work was supported by Autism Speaks (Basic and Clinical Grant #7618), the Stiftelsen Sunnerdahls Handikappfond Foundation, the Swedish Regional agreement on medical training and clinical research (ALF) and the Swedish Research Council (Grant # 2012-2264). The data linkages and staff costs have also been supported by grants from the Stockholm County Council (Grant # 2007008); Swedish Council for Working Life and Social Research (Grant # 2007–2064); Swedish Research Council (Grant # 523-2010-1052); and Swedish Regional agreement on medical training and clinical research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
PowerPoint slides
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Kosidou, K., Dalman, C., Widman, L. et al. Maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring: a population-based nationwide study in Sweden. Mol Psychiatry 21, 1441–1448 (2016). https://doi.org/10.1038/mp.2015.183
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.183
This article is cited by
-
Elevated circulating adiponectin levels do not prevent anxiety-like behavior in a PCOS-like mouse model
Scientific Reports (2024)
-
Associations between maternal metabolic conditions and neurodevelopmental conditions in offspring: the mediating effects of obstetric and neonatal complications
BMC Medicine (2023)
-
Associations between neighborhood stress and maternal sex steroid hormones in pregnancy
BMC Pregnancy and Childbirth (2023)
-
Steroid hormone pathways, vitamin D and autism: a systematic review
Journal of Neural Transmission (2023)
-
Prenatal Androgen Exposure and Traits of Autism Spectrum Disorder in the Offspring: Odense Child Cohort
Journal of Autism and Developmental Disorders (2023)