Abstract
Streptococcus pyogenes (also known as group A Streptococcus, GAS), the agent of streptococcal sore throat and invasive soft-tissue infections, attaches to human pharyngeal or skin epithelial cells through specific recognition of its hyaluronic acid capsular polysaccharide by the hyaluronic-acid-binding protein CD44 (refs 1, 2). Because ligation of CD44 by hyaluronic acid can induce epithelial cell movement on extracellular matrix3,4,5, we investigated whether molecular mimicry by the GAS hyaluronic acid capsule might induce similar cellular responses. Here we show that CD44-dependent GAS binding to polarized monolayers of human keratinocytes induced marked cytoskeletal rearrangements manifested by membrane ruffling and disruption of intercellular junctions. Transduction of the signal induced by GAS binding to CD44 on the keratinocyte surface involved Rac1 and the cytoskeleton linker protein ezrin, as well as tyrosine phosphorylation of cellular proteins. Studies of bacterial translocation in two models of human skin indicated that cell signalling triggered by interaction of the GAS capsule with CD44 opened intercellular junctions and promoted tissue penetration by GAS through a paracellular route. These results support a model of host cytoskeleton manipulation and tissue invasion by an extracellular bacterial pathogen.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schrager, H. M., Albertí, S., Cywes, C., Dougherty, G. J. & Wessels, M. R. Hyaluronic acid capsule modulates M protein-mediated adherence and acts as a ligand for attachment of group A Streptococcus to CD44 on human keratinocytes. J. Clin. Invest. 101, 1708–1716 (1998).
Cywes, C., Stamenkovic, I. & Wessels, M. R. CD44 as a receptor for colonization of the pharynx by group A Streptococcus. J Clin. Invest. 106, 995–1002 (2000).
Lesley, F., Hyman, R. & Kincade, P. W. CD44 and its interaction with extracellular matrix. Adv. Immunol. 54, 271–335 (1993).
Oliferenko, S., Kaverina, I., Small, J. V. & Huber, L. A. Hyaluronic acid (HA) binding to CD44 activates Rac1 and induces lamellipodia outgrowth. J. Cell Biol. 148, 1159–1164 (2000); erratum J. Cell Biol. 149, 236 (2000).
Bourguignon, L. Y. W., Zhu, H., Shao, L. & Chen, Y.-W. CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid-dependent ovarian tumor cell migration. J. Biol. Chem. 276, 7327–7336 (2001).
Rheinwald, J. G. The role of terminal differentiation in the finite culture lifetime of the human epidermal keratinocyte. Int. Rev. Cytol. Suppl. 10, 25–33 (1979).
Rheinwald, J. G. Serial cultivation of normal human epidermal keratinocytes. Methods Cell Biol. 21A, 229–254 (1980).
Bourguignon, L. Y., Zhu, H., Shao, L. & Chen, Y. W. CD44 interaction with tiam1 promotes Rac1 signaling and hyaluronic acid-mediated breast tumor cell migration. J. Biol. Chem. 275, 1829–1838 (2000).
Tsukita, S., Oishi, K., Sato, N., Sagara, J. & Kawai, A. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J. Cell Biol. 126, 391–401 (1994).
Legg, J. W. & Isacke, C. M. Identification and functional analysis of the ezrin-binding site in the hyaluronan receptor, CD44. Curr. Biol. 8, 705–708 (1998).
Hall, A. Rho GTPases and the actin cytoskeleton. Science 279, 509–514 (1998).
Taher, T. E. et al. Signaling through CD44 is mediated by tyrosine kinases. Association with p56lck in T lymphocytes. J. Biol. Chem. 271, 2863–2867 (1996).
Parenteau, N. L., Bilbo, P., Nolte, C. J., Mason, V. S. & Rosenberg, M. The organotypic culture of human skin keratinocytes and fibroblasts to achieve form and function. Cytotechnology 9, 163–171 (1992).
Sabolinski, M. L., Alvarez, O., Auletta, M., Mulder, G. & Parenteau, N. L. Cultured skin as a ‘smart material’ for healing wounds: experience in venous ulcers. Biomaterials 17, 311–320 (1996).
Finlay, B. B. & Cossart, P. Exploitation of mammalian host cell functions by bacterial pathogens. Science 276, 718–725 (1997).
McCallum, S. J. & Theriot, J. A. in Cellular Microbiology (eds Cossart, P., Boquet, P., Normark, S. & Rappuoli, R.) 171–191 (ASM, Washington DC, 2000).
Ashbaugh, C. D., Warren, H. B., Carey, V. J. & Wessels, M. R. Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J. Clin. Invest. 102, 550–560 (1998).
Cline, P. R. & Rice, R. H. Modulation of involucrin and envelope competence in human keratinocytes by hydrocortisone, retinyl acetate, and growth arrest. Cancer Res. 43, 3203–3207 (1983).
Crowe, D. L., Hu, L., Gudas, L. J. & Rheinwald, J. G. Variable expression of retinoic acid receptor (RARβ) mRNA in human oral and epidermal keratinocytes; relation to keratin 19 expression and keratinization potential. Differentiation 48, 199–208 (1991).
Tchao, R. in Alternative Methods in Toxicology (ed. Goldberg, A. M.) 271–283 (Ann Liebert, New York, 1988).
McNamara, B. P. et al. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J. Clin. Invest. 107, 621–629 (2001).
Acknowledgements
We thank E. Meluleni for preparation of the histology specimens; M. Lowe for assistance with the confocal microscopy; and R. Stearns for assistance with scanning electron microscopy. This work was supported by the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Supplementary information

Figure 1 a,b
(JPG 36.24 KB)

Figure 1 c
(JPG 38.07 KB)
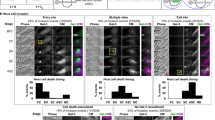
Confocal microscopy images demonstrating localization of keratinocyte-cell signaling molecules to the site of attachment of wild-type, but not acapsular, GAS. a, Samples were labeled with antibody to Rac1 (green) and to GAS (red). The left panels show merged images from the green and red channels. In the right panels, co-localization of green and red is shown in yellow: the images demonstrate co-localization of Rac1 at the site of attachment of wild-type GAS strain 950771 (cap+, arrows), but not acapsular mutant strain 188 (cap-). b, Samples were labeled with antibody to ezrin (green) and to GAS (red). The left panel shows merged images from the green and red channels. In the right panels, co-localization of green and red is shown in yellow: the images demonstrate co-localization of ezrin at the site of attachment of wild-type (cap+, arrows), but not acapsular (cap-), GAS. c, Samples were labeled with antibody to GAS (blue), to CD44 (red), and to phosphotyrosine (green), all of which are visible in the merged images in the left panels. In the other panels, co-localization of each of the potential fluorophore pairs is assessed: CD44 with phosphotyrosine appears yellow, GAS with CD44 appears pink, and GAS with phosphotyrosine appears blue. Co-localization of CD44 and phosphotyrosine is observed at the site of attachment to the keratinocyte of wild-type (cap+), but not acapsular (cap-), GAS.
Rights and permissions
About this article
Cite this article
Cywes, C., Wessels, M. Group A Streptococcus tissue invasion by CD44-mediated cell signalling. Nature 414, 648–652 (2001). https://doi.org/10.1038/414648a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/414648a
This article is cited by
-
Pathogenesis, epidemiology and control of Group A Streptococcus infection
Nature Reviews Microbiology (2023)
-
Complete genome sequences of Streptococcus pyogenes type strain reveal 100%-match between PacBio-solo and Illumina-Oxford Nanopore hybrid assemblies
Scientific Reports (2020)
-
Extracellular bacterial lymphatic metastasis drives Streptococcus pyogenes systemic infection
Nature Communications (2020)
-
Transcriptome analysis of human brain microvascular endothelial cells response to Neisseria meningitidis and its antigen MafA using RNA-seq
Scientific Reports (2019)
-
Membrane Dynamics in Health and Disease: Impact on Cellular Signalling
The Journal of Membrane Biology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.