Abstract
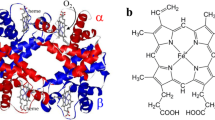
The atomic and electronic structures of heme complexes with His, Gly, and Cys residues (Heme–His, Heme–Gly, and Heme–Cys) in the fifth coordination position of the Fe atom and with oxygen and nitrogen oxide molecules in the sixth Fe position were studied by the semiempirical quantum-chemical method PM3. A comparative analysis of internuclear distances showed that the strength of chemical bonding between the ligand molecules (oxygen and nitrogen oxide) is greater for Heme–Cys than for Heme–His and Heme–Gly complexes. Consequently, the strengthening of the chemical bond of the oxygen (or nitrogen oxide) molecule with Heme–Cys substantially weakens the chemical bond in the ligand molecule. The Mulliken population analysis showed that the electronic density of ligand (oxygen or nitrogen oxide) p-orbitals is transferred to the d-orbitals of the Fe atom, whose charge, calculated according to the Mulliken analysis, formally becomes negative. In the Heme–His complex with oxygen, this charge is substantially greater than in the complex with NO, and the oxygen molecule becomes polarized. No oxygen polarization is observed in the Heme–Cys complex, and the electron density (judging from the change in the Fe charge) is transferred to the coordinated sulfur atom. This is also characteristic of Heme–Cys complexes with nitrogen oxide. An analysis of charges on the atoms indicates that the character of chemical bonding of the oxygen molecule in Heme–Cys and Heme–Gly complexes is similar and basically differs from that in the case of the Heme–His complex.
Similar content being viewed by others
REFERENCES
Cotton, F.A. and Wilkinson, G., Basic Inorganic Chemistry, New York: Wiley, 1976. Translated under the title Osnovy neorganicheskoi khimii, Moscow: Mir, 1979.
Watanabe, Y., 11th Int. Symp. Microsomes and Drug. Oxid., Los Angeles, CA, 1996, p. 84.
Balasubramanian, S., Lambright, D.G., Marden, M.C., and Boxer, S.G., Biochem., 1993, vol. 32, pp. 2202-2208.
Lambright, D.G., Balasubramanian, S., Decatur, S.M., and Boxer, S.G., Biochem., 1994, vol. 33, pp. 5518-5524.
Barrick, D., Biochem., 1994, vol. 33, pp. 6546-6552.
DePillis, G.D., Decatur, S.M., Barrick, D., and Boxer, S.G., J. Am. Chem. Soc., 1994, vol. 116, pp. 6981-6985.
Decatur, S.M. and Boxer, G.S., Biochem., 1995, vol. 34, pp. 2122-2127.
Antina, E.V., Lebedeva, N.Sh., and V'yugin, A.I., Zh. Koordin. Khim., 2001, vol. 27, pp. 784-789.
Mink, L.M., Polam, J.R., Christensen, K.A., Bruck, M.A., and Walker, F.A., J. Am. Chem. Soc., 1995, vol. 117, pp. 9329-9336.
Miller, L.M. and Chance, M.R., Biochem., 1995, vol. 34, pp. 10 170-10 174.
Jewsbury, P., Yamamoto, S., Minato, T., Saito, M., and Kitagawa, T., J. Phys. Chem., 1995, vol. 99, pp. 12 677-12 682.
Tokita, Y. and Nakatsuji, H., J. Phys. Chem., 1997, vol. 101, pp. 3281-3286.
Ghosh, A., Gonzales, E., and Vangberg, T., J. Phys. Chem., 1999, vol. 103, pp. 1363-1370.
Rovira, C., Kunc, K., Hutter, J., Ballone, P., and Parrinello, M., J. Phys. Chem., 1997, vol. 101, pp. 8914-8923.
Romanova, T., Krasnov, P., and Avramov, P., Absracts of Papers, XVI Int. Winterschool on Electronic Properties of Novel Materials: Molecular Nanoclusters, 2002, Kirchberg, Tirol, Austria, p. 74.
Romanova, T.A., Krasnov, P.O., Kachin, S.V., and Avramov, P.V., Teoriya i praktika komp'yuternogo modelirovaniya nanoob'ektov: Spravochnoe elektronnoe posobie (Theory and Practice of Modeling Nanosubjects: A Handbook), Registered in FGUP NTTs Informregistr, 2002, no. 0320200885.
Stewart, J.J.P., J. Comput. Chem., 1989, vol. 10, pp. 209-215.
Schmidt, M.W., Baldridge, K.K., Boatz, J.A., Elbert, S.T., Gordon, M.S., Jensen, J.H., Koseki, S., Matsunaga, N., Nguyen, K.A., Su, S.J., Windus, T.L., Dupuis, M., and Montgomery, J.A., J. Comput. Chem., 1993, vol. 14, pp. 1347-1363.
Romanova, T.A., Krasnov, P.O., and Avramov, P.V., Vopr. Med. Khim., 2001, vol. 47, pp. 308-315.
Romanova, T.A. and Avramov, P.V., Dokl. Ross. Akad. Nauk, 2002, vol. 383, pp. 116-119.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Romanova, T.A., Krasnov, P.O., Kuzubov, A.A. et al. The Role of Histidine in the Ligand-Bonding Capacity of the Hemoglobin Gene. Russian Journal of Bioorganic Chemistry 30, 124–128 (2004). https://doi.org/10.1023/B:RUBI.0000023096.21469.8e
Issue Date:
DOI: https://doi.org/10.1023/B:RUBI.0000023096.21469.8e