Abstract
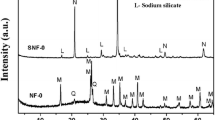
In order to synthesize Na-X zeolite from coal fly ash (Fa), Fa was pretreated under stirring condition at various temperatures of 20–50°C for 72 h and then aged at 85°C for a given period with NaOH solutions. The resulting materials were characterized by various means. When Fa was aged for 72 h without pretreatment, species P were formed. As the pretreating temperature raised from 20 to 50°C, the degree of crystallinity of faujasite increased, while that of species P decreased. The faujasite species formed was identified as Na-X zeolite with molar ratio SiO2/Al2O3 = 2.4. When Fa was pretreated at 50°C and aged for 60 h, the only species formed was Na-X zeolite. Increasing the pretreating temperature up to 50°C results in the increase of Si4+ and Al3+ concentrations in the treating solution by dissolution of amorphous material in Fa. With the conditions used, the crystalline phase, such as α-quartz and mullite, was poorly dissolved during the treatment. Hence, the higher pretreating temperature would give the uniform nucleation and crystal growth of Na-X zeolite during the aging.
Similar content being viewed by others
References
Coal Ash Handbook, Kankyo Gijyutsu Kyokai and Nippon Fly Ash Kyokai (2000).
A. A. Eleewi, A. L. Page and S. R. Geimn, J. Environ. Qual. 9 (1980) 242.
A. Boccaccini, M. Petimermet and E. Intermantel, Amer. Ceram. Soc. Bull. 76 (1997) 55.
T. Henmi, New Ceram. 7 (1997) 54.
Idem., Caly Sci. 6 (1987) 277.
Y. Okada, Jpn. J. Soil. Plant Nutri. 62 (1991) 1.
J. L. Larosa, S. Kwan and M. W. Grutzeck, J. Amer. Ceram. Soc. 75 (1992) 1574.
N. Shigemoto, H. Hayashi and K. Miura, J. Mater. Sci. 28 (1993) 4781.
H.-L. Chang and W.-H. Shih, Ind. Eng. Chem. Res. 39 (2000) 4185.
P. Kumar, Y. Oumi, T. Sano and K. Yamana, J. Ceram. Soc. Jpn. 109 (2001) 968.
M. Park and J. Choi, Clay Sci. 9 (1995) 219.
T. Henmi, Soil Sci. Plant Nutri. 33 (1987) 519.
H. Tanaka, S. Matsumura, S. Furusawa and R. Hino, J. Mater. Sci. Lett. 22 (2003) 323.
S. C. White and E. D. Case, J. Mater. Sci. 25 (1990) 5215.
E. Dempsey, G. H. Kuhl and D. H. Olson, J. Phys. Chem. 73 (1969) 387.
P. Catalfamo, F. Corigliano, P. Primerano and S. D. Pasqual, J. Chem. Soc., Faraday Trans. 89 (1993) 171.
E. M. Franigen, H. Khatami and H. A. Szymanski, “Molecular Sieve Zeolite I” (Academic, Prague, 1971) p. 201.
C.-F. Lin and H.-C. Shi, Environ. Sci. Technol. 29 (1995) 1109.
N. Murayama, H. Yamamoto and J. Shibata, Int. J. Minera. Process. 64 (2002) 1.
D. W. Breck, in “Zeolite Molecular Sieves” (John Wiley & Sons, New York, 1974).
H. Tanaka, Y. Sakai and R. Hino, Mater. Res. Bull. 37 (2002) 1873.
H. Tanaka, S. Furusawa and R. Hino, J. Mater. Syn. Process. 10 (2002) 43.
H. Tanaka, Y. Sakai and R. Hino, Proceedings of the 20th International Japan-Korea Seminar on Ceramics, 2003, 591.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, H., Matsumura, S. & Hino, R. Formation process of Na-X zeolites from coal fly ash. Journal of Materials Science 39, 1677–1682 (2004). https://doi.org/10.1023/B:JMSC.0000016169.85449.86
Issue Date:
DOI: https://doi.org/10.1023/B:JMSC.0000016169.85449.86