Abstract
1. The present work shows the results on behavior and on biochemical parameters of l-deprenyl (0.1, 5, and 10 mg/kg, p.o.) administered daily for 5 days to rats submitted to global cerebral ischemia.
2. The transient global ischemia was carried out by clamping the animals bilateral common carotid arteries for 20 min. The parameters studied were memory acquisition and memory retention, locomotor activity and thiobarbituric acid reactive substances, as an index of lipid peroxidation.
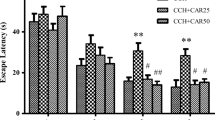
3. l-Deprenyl treatment significantly improved memory deficits as compared to the ischemic group as measured by the elevated T maze test. A similar result was observed on the passive avoidance test where l-deprenyl improved late but not early memory as compared to the ischemic group. Except for an increased locomotor activity observed in the group treated with 5 mg/kg, no other alteration was detected in this behavioral test. Rats submitted to transient global ischemia (and without l-deprenyl) showed an increase in MDA levels in the hippocampus and the treatment with l-deprenyl (5 or 10 mg/kg) significantly reversed this effect bringing values close to those of the sham-operated controls. A similar profile was observed with nitrite levels.
4. In conclusion, the work showed a significant protective effect of l-deprenyl on memory deficits and lipid hyperperoxidation observed after cerebral ischemia. Possibly, the drug is acting at least in part through its antioxidant activity.
Similar content being viewed by others
REFERENCES
Andersen, M. B., and Sams-Dodd, F. (1998). Impairment of working memory in the T-maze after transient global cerebral ischemia in the Mongolian gerbil. Behav. Brain Res. 91 (1/2):15-22
Archer, J. (1973). Tests for emotionality in rats and mice: A review. Anim. Behav. 21:205-235.
Barbelivien, A., Nyman, L., Haapalinna, A., and Sirvio, J. (2001). Inhibition of MAO-A activity enhances behavioral activity of rats assessed using water maze and open arena tasks. Pharmacol. Toxicol. 88:304-312.
Bederson, J. B., Pitts, L. H., Germano, S. M., Nishimura, M. C., Davis, R. L., and Bartkowski, H. M. (1986). Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 17:1304-1308.
Birks, J., and Flicker, L. (2000). Selegiline for Alzheimer's disease. Cochrane Database Syst Rev, CD 000442.
Brandeis, R., Sapir, M., Kapon, Y., Borelli, G., Cadel, S., and Valsecchi, B. (1991). Improvement of cognitive function by MAO-B inhibitor L-Deprenyl in aged rats. Pharmacol. Biochem. Behav. 39 (2):297-304.
Candelario-Jalil, E., Mhadu, N. H., Al-Dalain, S. M., Martinez, G., and Leon, O. S. (2001). Time course of oxidative damage in different brain regions following transient cerebral ischemia in gerbils. Neurosci. Res. 41:233-241.
De Vecchi, E., Lubatti, L., Beretta, C., Ferrero, S., Rinaldi, P., Kienle, M. G., Trazzi R., and Paroni, R. (1998). Protection from renal ischemia-reperfusion injury by the 2-methylaminochroman U83836E. Kidney Int. 54:857-863.
DeNoble, V. J., Repetti, S. J., Gelpke, L. W., Wood, L. M., and Keim, K. L. (1986). Vinpocetine: Nootropic effects on scopolamine-induced and hypoxia-induced retrieval deficits of a step-through passive avoidance response in rats. Pharmacol. Biochem. Behav. 24:1123-1128.
Dixit, S. N., Behari, M., and Ahuja, G. K. (1999). Effect of selegiline on cognitive functions in Parkinson's disease. J. Assoc. Physicians India 47:784-786.
Ebadi, M., Sharma, S., Shavali, S., and El Refaey, H. (2002). Neuroprotective actions of selegiline. J. Neurosci. Res. 67:285-289.
Goldlust, E. J., Paczynski, R. P., He, Y. Y., Hsu, C. Y., and Goldberg, M. P. (1996). Automated measurement of infarct size with scanned images of triphenyltetrazolium chloride-stained rat brains. Stroke 27:1657-1662.
Green, L. C., Tannenbaum, S. R., and Goldman, P. (1981). Nitrate synthesis in the germfree and conventional rat. Science 212:56-58.
Haba, K., Ogawa, N., Mizukawa, K., and Mori, A. (1991). Time course of changes in lipd peroxidation, pre-and postsynaptic cholinergic indices, NMDA receptor binding and neuronal death in the gerbil hippocampus following transient ischemia. Brain Res. 540:116-122.
Halliwell, B. (2001). Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drugs Aging 18:685-716.
He, Z., Ibayashi, S., Sugimori, H., Fujii, K., Sadoshima, S., and Fujishima, M. (1997). Age-related ischemia in the brain following bilateral carotid artery occlusion-collateral blood flow and brain metabolism. Neurochem. Res. 22:37-42.
Head, E., Hartley, J., Kameka, A. M., Mehta, R., Ivy, G. O., Ruehl, W. W., and Milgram, N. W. (1996). The effects of l-deprenyl on spatial short term memory in young and aged dogs. Prog. Neuropsychopharmacol. Biol. Psychiatry 20 (3):515-530.
Herguido, M. J., Carceller, F., Roda, J. M., and Avendano, C. (1999). Hippocampal cell loss in transient global cerebral ischemia in rats: A critical assessment. Neuroscience 93 (1):71-80.
Homi, H. M., Freitas, J. J., Curi, R., Velasco, I. T., and Junior, B. A. (2002). Changes in superoxide dismutase and catalase activities of brain regions during early global transient ischemia/reperfusion. Neurosci. Lett. 323:37-40.
Knoll, J. (1983). Deprenyl (selegiline): The history of its development and pharmacological action. Acta Neurol. Scan. 95 (Suppl):57-80.
Knoll, J., Miklya, I., Knoll, B., Marko, R., and Kelemen, K. (1996). Deprenyl and (-)-1-phenyl-2-propylaminopentane [(-)PPAP] act primarily as potent stimulants of action-potential-transmitter release coupling in the catecholaminergic neurons. Life Sci. 58:517-527.
Lahtinen, H., Koistinaho, J., Kauppinen, R., Haapalina, A., Keinanen, R., and Sevenius, J. (1997). Selegiline treatment after transient global ischemia in gerbils enhances the survival of CA1 pyramidal cells in the hippocampus. Brain Res. 757:260-267.
Magyar, K., Szende, B., Lengyel, J., and Tekes, K. (1996). The pharmacology of B-type selective monoamine oxidase inhibitors: Milestone in (-)-deprenyl research. J. Neurol. Transm. 48 (Suppl):29-43.
Maruyama, W., Takahashi, T., and Naoi, M. (1998). (-)-Deprenyl protects human dopaminergic neuroblastoma SH-SY5Y cells from apoptosis induced by peroxynitrite and nitric oxide. J. Neurochem. 70 (6):2510-2515.
Mathews, K. S., McLaughlin, D. P., Ziabari, L. H., Toner, C. C., Street, P. C., Hisgrove, E., Lindsey, Bezzina, E. L., and Stanford, J. A. (2000). Rapid quantification of ischemic injury and cerebroprotection in brain slices using densitometric assessment of 2,3,5-triphenyltetrazolium chloride staining. J. Neurosci. Methods 102:43-51.
Mazzio, E., Huber, J., Darling, S., Harris, N., and Soliman, K. F. (2001). Effect of antioxidants on L-glutamate and N-methyl-4-phenylpyridinium ion induced-neurotoxicity in PC12 cells. Neurotoxicology 22:283-288.
Mihara, M., and Uchiyama, M. (1978). Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 86 (1):271-278.
Nowakowska, E., Kus, K., Chodera, A., and Rybakowski, J. (2001). Investigating potential anxiolytic, antidepressant and memory enhancing activity of deprenyl. J. Physiol. Pharmacol. 52 (4 Pt 2):863-873.
Paller, M. S. (1994). The cell biology of reperfuison injury in the kidney. J. Invest. Med. 42:632-639.
Paxinos, G., and Watson, C. (1986). The Rat Brain in Stereotaxic Coordinates. 2nd ed. Sydney: Academic Press
Perez-Pinzon, M. A., Sick, T. J., Rosenthal, M. (1999). Mechanisms of mitochondrial hyperoxidation after global cerebral ischemia. Adv. Exp. Med. Biol. 471:175-180.
Puurunen, K., Jolkkonen, J., Sirvio, J., Haapalinna, A., and Sivenius, J. (2001). Selegiline combined with enriched-environment housing attenuates spatial learning deficits following focal cerebral ischemia in rats. Exp. Neurol. 167:348-355.
Tatton, W. G., Wadia, J. S., Ju, W. Y., Chalmers-Redman, R. M., and Tatton, N. A. (1996). (-)-Deprenyl reduces neuronal apoptosis and facilitates neuronal outgrouth by altering protein synthesis without inhibitory monoamine oxidase. J. Neural. Transm. 48 (Suppl):45-59.
Thomas, T. (2000). Monoamine oxidase-B-inhibitors in the treatment of Alzheir's disease. Neurobiol. Aging. 21:343-348.
Una, I., Gursoy-Ozdemir, Y., Bolay, H., Soylemezoglu, F., Saribas, O., and Dalkara, T. (2001). Chronic daily administration of selegiline and Egb 761 increases brain's resistance to ischemia in mice. Brain Res. 917:174-181.
Viana, M. B., Tomaz, C., and Graeff, F. G. (1994). The elevated T-Maze: A new animal model of anxiety and memory. Pharmacol. Biochem. Behav. 49:549-554.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maia, F.D., Pitombeira, B.S.S., Araújo, D.T. et al. l-Deprenyl Prevents Lipid Peroxidation and Memory Deficits Produced by Cerebral Ischemia in Rats. Cell Mol Neurobiol 24, 87–100 (2004). https://doi.org/10.1023/B:CEMN.0000012727.59502.c5
Issue Date:
DOI: https://doi.org/10.1023/B:CEMN.0000012727.59502.c5