Abstract
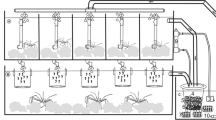
Scallop farms in Norway rely exclusively on hatchery production of spat. Larval rearing is one of the most difficult parts of the production, and several experiments have been performed during the last years to improve the larval systems. This paper describes results from commercial and experimental trials obtained between 1996 and 2001. Four different rearing systems were compared: untreated batch cultures, chloramphenicol-treated batch cultures, flow-through cultures with filtered water and flow-through cultures with water from a biofilter. The chloramphenicol-treated batch cultures had overall significantly higher survival rates than the untreated and flow-through cultures. There were no significant differences in survival between untreated and flow-through cultures. The average survival rates for the untreated, treated and flow-through cultures were 6.8, 23.0 and 8.6%, respectively. No significant seasonal differences were found for the untreated and treated larval groups, whereas for larvae reared in flow-through systems survival was significantly higher in the winter than the spring. Overall best results were obtained during winter in years with high salinity water in the fjord.
Similar content being viewed by others
References
Aiken D. 1993. Cada vez hay más y más ostiónes! World Aquaculture 24: 7-19.
Andersen S. and Ringvold H. 2000. Seasonal differences in effect of broodstock diet on spawning success. Aquaculture International 8: 259-265.
Andersen S., Burnell G. and Bergh Ø. 2000. Flow-through systems for culturing great scallop larvae. Aquaculture International 8: 249-257.
Anonymous 1990. Council regulation (EEC) No. 2377/90 (1990). The European Union.
Anonymous 1991. Training manual on breeding and culture of scallop and sea cucumber in China. Yellow Sea Fisheries Research Institute in Qingdao, People's Republic of China and Regional Seafarming Development and Demonstration Project (RAS/90/002), Qingdao, China, RAS/90/002, 84 pp. Training manual 9.
Anonymous 1994. Commission regulation (EC) No. 2701/94 (1994). The European Union.
Aure J. and Strand Ø. 2001. Hydrographic normals and long-term variations at fixed surface layer stations along the Norwegian coast from 1936 to 2000. Fisken og Havet. Institute of Marine Research, Bergen, Norway, 13, 26 pp.
Beal B.F. and Chapman S.R. 2001. Methods for mass rearing stages i–iv larvae of the American lobster, Homarus americanus H. Milne Edwards, 1837, in static systems. Journal of Shellfish Research 20: 337-346.
Bergh Ø. and Strand Ø. 2001. Great scallop, Pecten maximus, research and culture strategies in Norway: a review. Aquaculture International 9: 305-318.
Cochard J.C. and Devauchelle N. 1993. Spawning, fecundity and larval survival and growth in relation to controlled conditioning in native and transplanted populations of Pecten maximus (L) — evidence for the existence of separate stocks. Journal of Experimental Marine Biology and Ecology 169: 41-56.
Cochard J.C. and Gerard A. 1987. Production artificielle de naissain de coquilles Saint-Jacques Pecten maximus (L.) en rade de Brest: analyse des facteurs affectant la croissance larvaire. Menai Bridge, Wales, p. 13.
Cruz P. and Ibarra A.M. 1997. Larval growth and survival of two Catarina scallop (Argopecten circularis, Sowerby, 1835) populations and their reciprocal crosses. Journal of Experimental Marine Biology and Ecology 212: 95-110.
Duinker A., Saout C. and Paulet Y.M. 2000. Effect of photoperiod on conditioning of the great scallop. Aquaculture International 7: 449-457.
Gallager S.M. and Mann R. 1986. Growth and survival of larvae of Mercenaria mercenaria (L.) and Crassostrea virginica (Gmelin) relative to broodstock conditioning and lipid content of eggs. Aquaculture 56: 105-121.
Gerard A., Salaun M. and Tritar S. 1989. Criteria of larval competence to metamorphosis in Pecten maximus. Haliotis 19: 373-380.
Gruffyd L.L.D. and Beaumont A.R. 1970. Determination of the optimum concentration of eggs and spermatozoa for the production of normal larvae in Pecten maximus (Mollusca, Lamellibranchia). Helgoländer wiss. Meeresunters 20: 486-497.
Harboe T., Tuene S., Mangor-Jensen A., Rabben H. and Huse I. 1994. Design and opera-tion of an incubator for yolk-sac larvae of Atlantic halibut. Progressive Fish-Culturist 56: 188-193.
Harboe T., Mangor-Jensen A., Naas K.E. and Næss T. 1998. A tank design for first feeding of Atlantic halibut, Hippoglossus hippoglossus L., larvae. Aquaculture Research 29: 919-923.
Ibarra A.M., Ramirez J.L. and Garcia G.A. 1997. Stocking density effects on larval growth and survival of two catarina scallop, Argopecten ventricosus (= circularis) (Sowerby II, 1842), populations. Aquaculture Research 28: 443-451.
Lambert C., Nicolas J.L., Cilia V. and Corre S. 1998. Vibrio pectenicida sp. nov., a pathogen of scallop (Pecten maximus) larvae. International Journal of Systematic Bacteriology 48: 481-487.
Lee M.J., Taylor G.T., Bricelj V.M., Ford S.E. and Zahn S. 1996. Evaluation of Vibri o spp. and microplankton blooms as causative agents of juvenile oyster disease in Crassostrea virginica (Gmelin). Journal of Shellfish Research 15: 319-330.
Leibovitz L. 1989. Chlamydiosis: a newly reported serious disease of larval and postmetamorphic bay scallops, Argopecten irradians (Lamarck). Journal of Fish Diseases 12: 126-136.
Nicolas J.L., Corre S., Robert R. and Ansquer D. 1995. Why do scallop (Pecten maximus) larvae die, when they are reared without antibiotic? 10th International Pectinid Workshop, Book of abstracts April 27–May 2 1995 Cork, Ireland, pp. 53-54.
Nicolas J., Corre S., Gauthier G., Robert R. and Ansquer D. 1996. Bacterial problems associated with scallop Pecten maximus larval culture. Diseases of Aquatic Organisms 27: 67-76.
Nicosia F. and Lavalli K. 1999. Homarid Lobster Hatcheries: their history and role in research, management, and aquaculture. Marine Fisheries Review 61: 1-57.
Nightingale W.H. 1936. Red water organisms: their occurrence and influence upon marine aquatic animals. With special reference to shellfish in waters of the Pacific coast. Argus Press, Seattle, USA, 24 pp.
O'Connor W.A. and Heasman M.P. 1998. Ontogenetic changes in salinity and temperature tolerance in the doughboy scallop, Mimachlamys asperrima. Journal of Shellfish Research 17: 89-95.
Prickett R.A. and Iakovopoulos G. 1996. Potential gains through careful management of current technology. In: Chatain B., Saroglia B., Sweetman J. and Lavens P. (eds.), European Aquaculture Society, Verona, Italy, pp. 206-211.
Riquelme C., Hayashida G., Toranzo A.E., Vilches J. and Chavez P. (1995) Pathogenicity studies on a Vibrio anguillarum-related (var) strain causing an epizootic in Argopecten purpuratus larvae cultured in Chile. Diseases of Aquatic Organisms 22: 135-141.
Robert R. and Gérard A. 1999. Bivalve hatchery technology: the current situation for the Pacific oyster Crassostrea gigas and the scallop Pecten maximus in France. Aquatic Living Resources 12: 121-130.
Robert R., Miner P. and Nicolas J.L. 1996. Mortality control of scallop larvae in the hatchery. Aquaculture International 4: 305-313.
Ruiz C.M., Román G. and Sánchez J.L. 1995. Effect of Three Different Marine Bacteria Strains on Larval Cultures of Pecten maximus. Cork, Ireland, pp. 113-114.
Serfling S.A., Van Olst J.C. and Ford R.F. 1974. A recirculating culture system for larvae of the American lobster, Homarus americanus. Aquaculture 3: 303-309.
Shields R.J. 2001. Larviculture of marine finfish in Europe. Aquaculture 200: 55-88.
Siegel S. and Castellan N.J. 1988. Nonparametric Statistics for the Behavioural Science. McGraw-Hill Book Company, New York, 399 pp.
Sokal R.R. and Rohlf F.J. 1981. Biometry. The Principle and Practice of Statistics in Biological Research. W.H. Freeman and Company, New York, 859 pp.
Southgate P.C. and Ito M. 1998. Evaluation of a partial flow-through culture technique for pearl oyster (Pinctada margaritifera L.) larvae. Aquaculture Engineering 18: 1-7.
Strand Ø. and Nylund, A. 1991. The reproductive cycle of the scallop Pecten maximus (Linnaeus, 1758) from two populations in western Norway, 60°N and 64°N. In: Shumway S.E. (ed.), An International Compendium of Scallop Biology and Culture. World Aquaculture Society, pp. 95-105.
Strand Ø. and Vølstad J.H. 1997. The molluscan fisheries and culture of Norway. U.S. Department of Commerce, NOAA Technical Report NMFS 129, pp. 7-24.
Strohmeier T., Duinker A. and Lie Ø. 2000. Seasonal variations in chemical composition of the female gonad and storage organs in Pecten maximus (L.) Suggesting that somatic and reproductive growth are separated in time. Journal of Shellfish Research 19: 741-747.
Taylor J.E., Gunn B., Williams P. and McNicoll I. 1994. An investigation of techniques for the hatchery and nursery rearing of the king scallop, Pecten maximus (L.). Bourne N. and Bunting B. (eds.), Proceedings of the 9th International Pectinid Workshop, 22–27 April 1993, Nanaimo, B.C., Canada, Townsend, LD, pp. 104-108.
Torkildsen L., Samuelsen O.B., Lunestad B.T. and Bergh Ø. 2000. Minimum inhibitory con-centrations of chloramphenicol, florfenicol, trimethoprim/sulfadiazine and flumequine in seawater of bacteria associated with scallops (Pecten maximus) larvae. Aquaculture 185: 1-12.
Uriarte I., Farias A. and Castilla J.C. 2001. Effect of antibiotic treatment during larval development of the Chilean scallop, Argopecten purpuratus. Aquacultural Engineering 25: 139-147.
Wikfors GH. and Smolowitz R. 1994. Dinoflagellate autolysosomes and responses of different bivalves feeding on Prorocentrum — is there a connection? 14. Annual Milford Aquaculture Seminar, Milford, CT, USA, 22–24 February 1994. Milford, USA. Journal of Shellfish Research, pp. 322-323.
Yan T., Zhou M.J., Fu M., Wang Y.F., Yu R.C. and Li J. 2001. Inhibition of egg hatching success and larvae survival of the scallop, Chlamys farreri, associated with exposure to cells and cell fragments of the dinoflagellate Alexandrium tamarense. Toxicon 39: 1239-1244.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Torkildsen, L., Magnesen, T. Hatchery Production of Scallop Larvae (Pecten maximus) – Survival in Different Rearing Systems. Aquaculture International 12, 489–507 (2004). https://doi.org/10.1023/B:AQUI.0000042143.53903.21
Issue Date:
DOI: https://doi.org/10.1023/B:AQUI.0000042143.53903.21