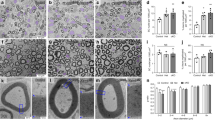
Abstract
Fish optic nerve fibres quickly regenerate after injury, but the onset of remyelination is delayed until they reach the brain. This recapitulates the timetable of CNS myelinogenesis during development in vertebrate animals generally, and we have used the regenerating fish optic nerve to obtain evidence that it is the axons, not the myelinating glial cells, that determine when myelin formation begins. In fish, the site of an optic nerve injury becomes remyelinated by ectopic Schwann cells of unknown origin. We allowed these cells to become established and then used them as reporters to indicate the time course of pro-myelin signalling during a further round of axonal outgrowth following a second upstream lesion. Unlike in the mammalian PNS, the ectopic Schwann cells failed to respond to axotomy and to the initial outgrowth of new optic axons. They only began to divide after the axons had reached the brain. Shortly afterwards, small numbers of Schwann cells began to leave the dividing pool and form myelin sheaths. More followed gradually, so that by 3 months remyelination was almost completed and few dividing cells were left. Moreover, remyelination occurred synchronously throughout the optic nerve, with the same time course in the pre-existing Schwann cells, the new ones that colonised the second injury, and the CNS oligodendrocytes elsewhere. The optic axons are the only common structures that could synchronise myelin formation in these disparate glial populations. The responses of the ectopic Schwann cells suggest that they are controlled by the regenerating optic axons in two consecutive steps. First, they begin to proliferate when the growing axons reach the brain. Second, they leave the cell cycle to differentiate individually at widely different times during the ensuing 2 months, during the critical period when the initial rough pattern of axon terminals in the optic tectum becomes refined into an accurate map. We suggest that each axon signals individually for myelin ensheathment once it completes this process.
Similar content being viewed by others
References
BAICHWAL, R. R. & DEVRIES, G. H. (1989) A mitogen for Schwann cells is derived from myelin basic protein. Biochemical and Biophysical Research Communications 164, 883–888.
BARRES, B. A., LAZAR, M. A. & RAFF, M. C. (1994) A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 120, 1097–1108.
BEAZLEY, L. D., SHEARD, P. W., TENNANT, M., STARAC, D. & DUNLOP, S. A. (1997) Optic nerve regenerates but does not restore topographic projections in the lizard Ctenophorus ornatus. Journal of Comparative Neurology 377, 105–120.
BENOWITZ, L. I. & SCHMIDT, J. T. (1987) Activity dependent sharpening of the regenerating retinotectal projection in goldfish: Relationship to the expression of growthassociated proteins. Brain Research 417, 118–126.
BERRY, M., HUNTER, S. A., DUNCAN, A., LORDAN, J., KIRVELL, S., TSANG, W. L. & BUTT, A. M. (1998) Axon-glial relations during regeneration of axons in the adult rat anterior medullary velum. Journal of Neurocytology 27, 915–937.
BLAKEMORE, W. F., EAMES, R. A., SMITH, K. J. & MCDONALD, W. I. (1977) Remyelination in the spinal cord of the cat following intraspinal injections of lysolecithin. Journal of the Neurological Sciences 33, 31–43.
BLAKEMORE, W. F., & FRANKLIN, R. J. (1991) Transplantation of glial cells into the CNS. Trends in Neuroscience 14, 323–327.
BLAKEMORE, W. F. & PATTERSON, R. C. (1975) Observations on the interactions of Schwann cells and astrocytes following X-irradiation of neonatal rat spinal cord. Journal of Neurocytology 4, 573–585.
BUNGE, R. P. (1987) Tissue culture observation relevant to the study of axon-Schwann cell interactions during peripheral nerve development and repair. Journal of Experimental Biology 132, 21–34.
CARTER, D. A., BRAY, G. M. & AGUAYO, A. J. (1989) Regenerated retinal ganglion cell axons can form welldifferentiated synapses in the superior colliculus of adult hamsters. Journal of Neuroscience 9, 4042–4050.
CELLERINO, A., CARROLL, P., THOENEN, H. & BARDE, Y. A. (1997) Reduced size of retinal ganglion cell axons and hypomyelination in mice lacking brainderived neurotrophic factor. Molecular and Cellular Neuroscience 9, 397–408.
CLINE, H. T. & CONSTANTINE-PATON, M. (1990) NMDA receptor agonist and antagonists alter retinal ganglion cell arbor structure in the developing frog retinotectal projection. Journal of Neuroscience 10, 1197–1216
CONSTANTINE-PATON, M., CLINE, H. T. & DEBSKI, E. (1990) Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annual Review of Neuroscience 13, 129–154.
COOK, J. E. (1987) A sharp retinal image increases the topographic precision of the goldfish retinotectal projection during optic nerve regeneration in stroboscopic light. Experimental Brain Research 68, 319–328.
COOK, J. E. (1990) Morphological recovery of axotomized goldfish retinal cells in an environmentknownto prevent retinotopic refinement of their regenerated tectal arbors. Brain Res. 510, 181–189.
COOK, J. E. & RANKIN, E. C. (1986) Impaired refinement of the regenerated retinotectal projection of the goldfish in stroboscopic light: A quantitative WGA-HRP study. Experimental Brain Research 63, 421–430.
DOWDING, A. J., MAGGS, A. & SCHOLES, J. (1991) Diversity amongst the microglia in growing and regenerating fish CNS: Immunohistochemical characterization using FL. 1, an anti-macrophage monoclonal antibody Glia. 4, 345–364.
FUCHS, C., GLASGOW, E., HITCHCOCK, P. F. & SCHECHTER, N. (1994) Plasticin, a newly identified neurofilament protein, is preferentially expressed in young retinal ganglion cells in adult goldfish. Journal of Comparative Neurology 350, 452–462.
GLASGOW, E., DRUGER, R. K., LEVINE, E. M., FUCHS, C. & SCHECHTER, N. (1992) Plasticin, a novel Type III neurofilament protein from goldfish retina: Increased expression during optic nerve regeneration. Neuron 9, 373–381.
HAMANO, K., TAKEYA, T., IWASAKI, N., NAKAYAMA, J., OHTO, T. & OKADA, Y. (1998) A quantitative study of the progress of myelination in the rat central nervous system using the immunohistochemical method for proteolipid protein. Developmental Brain Research 108, 287–293.
JESERICH, G. & RAUEN, T. (1990) Cell cultures enriched in oligodendrocytes from the central nervous system of trout in terms of phenotypic expression exhibit parallels with cultured rat Schwann cells. Glia 3, 65–74.
JESERICH, G. & WAEHNELDT, T. V. (1986) Characterisation of antibodies against major fish CNS myelin proteins: Immunoblot analysis and immunochemical localisation of 36K & IP2 proteins in trout nerve tissue. Journal of Neuroscience Research 15, 147–157.
JESSEN, K. R., BRENNAN, A., MORGAN, L., MIRSKY, R., KENT, A., HASHIMOTO, Y. & GAVRILOVIC, J. (1994) The Schwann cell precursor and its fate: A study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron 12, 509–527.
JESSEN, K. R. & MIRSKY, R. (1998) Origin and early development of Schwann cells. Microscopical Research and Technique 41, 393–402.
JESSEN, K. R. & MIRSKY, R. (1999) Developmental regulation in the Schwann cell lineage. Advances in Experimental and Medical Biology 468, 3–12.
KINNEY, H. C., BRODY, B. A., KLOMAN, A. S. & GILLES, F. H. (1988) Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. Journal of Neuropathology and Experimental Neurology 47, 217–234.
LINDHOLM, D., HEUMANN, R., MEYER, M. & THOENEN, H. (1987) Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature 330, 658–659.
LIVESEY, F. J., OÕBRIEN, J. A., LI, M., SMITH, A. G., MURPHY, L. J. & HUNT, S. P. (1997) A Schwann cell mitogen accompanying regeneration of motor neurons. Nature 390, 614–618.
LOONEY, G. A. & ELBERGER, A. J. (1986) Myelination of the corpus callosum in the cat: Time course, topography, and functional implications. Journal of Comparative Neurology 248, 336–347.
MAGGS, A. & SCHOLES, J. (1990) Reticular astrocytes in the fish optic nerve: Macroglia with epithelial characteristics form an axially repeated lacework pattern, to which nodes of Ranvier are apposed. Journal of Neuroscience 10, 1600–1614.
MEYER, R. L. & KAGEYAMA, G. H. (1999) Large-scale errors during map formation by regenerating axons in the goldfish. Journal of Comparative Neurology 409, 299–312.
MIRSKY, R. & JESSEN, K. R. (1999) The neurobiology of Schwann cells. Brain Pathology 9, 293–311.
MORRISSEY, T. K., LEVI, A. D., NUIJENS, A., SLIWKOWSKI, M. X. & BUNGE, R. P. (1995) Axoninduced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proceedings of the National Academy of Sciences U.S.A. 92, 1431–1435.
MUNZ, M., RASMINSKY, M., AGUAYO, A. J., VIDAL SANCHEZ, M. & DEVOR, M. G. (1985) Functional activity of rat brainstem neurons regenerating axons along peripheral nerve grafts. Brain Research 34, 115–125.
NONA, S. N. (1998) Repair in goldfish central nervous system. Restorative Neurology and Neuroscience 12, 1–11.
NONA, S. N., DUNCAN, A., STAFFORD, C. A., MAGGS, A., JESERICH, G. & CRONLY-DILLON, J. R. (1992) Myelination of regenerated axons in goldfish optic nerve by Schwann cells. Journal of Neurocytology 21, 391–401.
NONA, S. N., SHEHAB, S. A. S., STAFFORD, C. A. & CRONLY-DILLON, J. R. (1989) Glial fibrillary acidic protein (GFAP) from goldfish: Its localisation in the visual pathway. Glia 2, 189–200.
NONA, S. N. & STAFFORD, C. A. (1995) Glial repair at the lesion site in regenerating goldfish spinal cord: An immunohistochemical study using species-specific antibodies. Journal of Neuroscience Research 42, 350–356.
NONA, S. N., STAFFORD, C. A., SHEHAB, S. A. S. & CRONLY-DILLON, J. R. (1990) A polyclonal antibody to goldfish neuronal 145 kDa intermediate filament protein. Brain Research 524, 133–138.
NONA, S. N., THOMLINSON, A. M. & STAFFORD, C. A. (1998) Temporary colonization of the site of lesion by macrophages is a prelude to the arrival of regenerated axons in injured goldfish optic nerve. Journal of Neurocytology 27, 791–803.
OLSON, M. D. & MEYER, R. L. (1994) Normal activitydependent refinement in a compressed retinotectal projection in goldfish. Journal of Comparative Neurology 347, 481–494.
OPPENHEIM, R. W. (1991) Cell death during development of the nervous system. Annual Review of Neuroscience 14, 453–501.
PELLEGRINO, R. G. & SPENCER, P. S. (1985) Schwann cell mitosis in response to regenerating peripheral axons in vivo. Brain Research 341, 16–25.
PETERS, A., PALAY, S. & WEBSTER, H. DE. F. (1991) The Fine Structure of the Nervous System: The Neurons and the Supporting Cells, 3rd ed. New York: Oxford University Press.
POLITIS, M. J. & SPENCER, P. S. (1981) A method to separate spatially the temporal sequence of axon-Schwann cell interaction during development. Journal of Neurocytology 10, 221–231.
RAFF, M. C., DURAND, B. & GAO, F.-B. (1998) Cell number control and timing in animal development: The oligodendrocyte lineage. International Journal of Developmental Biology 42, 263–267.
RAGER, G. H. (1980) Development of the retinotectal projection in the chicken. Advances in Anatomy, Embryology and Cell Biology 63, 1–90.
RANKIN, E. C. & COOK, J. E. (1986) Topographic refinement of the regenerating retinotectal projection of the goldfish in standard laboratory conditions: A quantitative WGA-HRP study. Experimental Brain Research 63, 409–420.
REICH, J. B., BURMEISTER, D. W., SCHMIDT, J. T. & GRAFSTEIN, B. (1990) Effect of conditioning lesions on regeneration of goldfish optic axons: Time course of the cell body reaction to axotomy. Brain Research 515, 256–260.
REINIS, S. & GOLDMAN, M. (1980) The Development of the Brain. Illinois: Charles Thomas.
SCHERER, S. S. & EASTER, S. S. (1984) Degnerative and regenerative changes in the trochlear nerve of goldfish. Journal of Neurocytology 13, 519–565.
SCHMIDT, J. T. (1985) Formation of retinotopic connections: Selective stabilisation by an activity-dependent mechanism. Cellular and Molecular Neurobiology 5, 65–84.
SCHMIDT, J. T. (1990) Long term potentiation and activitydependent retinotopic sharpening in the regenerating retinotectal projection of goldfish: Common sensitive period and sensitivity to NMDA blockers. Journal of Neuroscience 10, 233–246.
SCHMIDT, J. & COEN, T. (1995) Changes in retinal arbors in compressed projections to half tecta in goldfish. Journal of Neurobiology 28, 409–418.
SCHMIDT, J. T. & EISELE, L. E. (1985) Stroboscopic illumination and dark rearing block the sharpening of the regenerated retinotectal map in goldfish. Neuroscience 14, 535–546.
SCHOLES, J. (1991) The design of the optic nerve in fish. Visual Neuroscience 7, 129–139.
SCHREYER, D. J. & JONES, E. G. (1982) Growth and target finding by axons in the corticospinal tract in prenatal and postnatal rats. Neuroscience 7, 1837–1853.
SCHWAB, M. E. & SCHNELL, L. (1989) Region-specific appearance of myelin constituents in the developing rat spinal cord. Journal of Neurocytology 18, 161–169.
SEFTON, A. J. & LAM, K. (1984) Quantitative and morphological studies on developing optic axons in normal and enucleated albino rats. Experimental Brain Research 57, 107–117.
SO, K. F. & AGUAYO, A. J. (1985) Lengthy regrowth of cut axons from ganglion cells after peripheral nerve transplantation into the retina of adult rats. Brain Research 382, 349–354.
SPEIDEL, C. C. (1964) In vivo studies of myelinated nerve fibres. International Review of Cytology 16, 173–231.
TEMPLE, S. & RAFF, M. C. (1986) Clonal analysis of oligodendrocyte development in culture: Evidence for a developmental clock that counts cell divisions. Cell 44, 773–779.
VOYVODIC, J. T. (1989) Target size regulates calibre and myelination of sympathetic axons. Nature 342, 430–433.
WANG, S., SDRULLA, A. D., DISIBIO, G., BUSH, G., NOFZIGER, D., HICKS, C., WEINMASTER, G. & BARRES, B. A. (1998) Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 21, 63–75.
WEBSTER, H. DE. F. (1993) In Peripheral Neuropathy (edited by DYCK, P. J., THOMAS, P. K. et al.) pp. 243–266. Philadelphia: W. B. Saunders.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nona, S., Thomlinson, A., Bartlett, C. et al. Schwann cells in the regenerating fish optic nerve: Evidence that CNS axons, not the glia, determine when myelin formation begins. J Neurocytol 29, 285–300 (2000). https://doi.org/10.1023/A:1026575805331
Issue Date:
DOI: https://doi.org/10.1023/A:1026575805331