Abstract
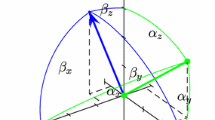
Residual dipolar couplings measured in weakly aligning liquid-crystalline solvent contain valuable information on the structure of biomolecules in solution. Here we demonstrate that dipolar couplings (DCs) can be used to derive a comprehensive set of pairwise angular restraints that do not depend on the orientation of the alignment tensor principal axes. These restraints can be used to assess the agreement between a trial protein structure and a set of experimental dipolar couplings by means of a graphic representation termed a `DC consistency map'. Importantly, these maps can be used to recognize structural elements consistent with the experimental DC data and to identify structural parameters that require further refinement, which could prove important for the success of DC-based structure calculations. This approach is illustrated for the 42 kDa maltodextrin-binding protein.
Similar content being viewed by others
References
Annila, A., Aitio, H., Thulin, E. and Drakenberg, T. (1999) J. Biomol. NMR, 14, 223–230.
Baber, J.L., Libutti, D., Levens, D. and Tjandra, N. (1999) J. Mol. Biol., 289, 949–962.
Bax, A. and Tjandra, N. (1997) J. Biomol. NMR, 10, 289–292.
Bayer, P., Varani, L. and Varani, G. (1999) J. Biomol. NMR, 14, 149–155.
Bloembergen, N. and Rowland, J.A. (1953) Acta Met., 1, 731.
Bolon, P.J., Al-Hashimi, H.M. and Prestegard, J.H. (1999) J. Mol. Biol., 293, 107–115.
Brünger, A.T. (1993) X-PLOR, A System for X-ray Crystallography and NMR, Yale University Press, New Haven, CT.
Brünger, A.T., Adams, P.D., Clore, G.M., Delano, W.L., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.S., Kuszewski, J., Nilges, M., Pannu, N.S., Read, R.J., Rice, L.M., Simonson, T. and Warren, G.L. (1998) Acta Crystallogr., D54, 905–921.
Byrd, P.F. and Friedman, M.D. (1954) Handbook of Elliptic Integrals for Engineers and Physicists, Springer-Verlag, Berlin.
Cai, M.L., Wang, H., Olejniczak, E.T., Meadows, R.P., Gunasekera, A. H., Xu, N. and Fesik, S.W. (1999) J. Magn. Reson., 139, 451–453.
Clore, G.M., Gronenborn, A.M. and Tjandra, N. (1998a) J. Magn. Reson., 131, 159–162.
Clore, G.M., Gronenborn, A.M. and Bax, A. (1998b) J. Magn. Reson., 133, 216–221.
Clore, G.M. and Garrett, D.S. (1999) J. Am. Chem. Soc., 121, 9008–9012.
Delaglio, F., Kontaxis, G. and Bax, A. (2000) J. Am. Chem. Soc., 122, 2142–2143.
Drohat, A.C., Tjandra, N., Baldisseri, D.M. and Weber, D.J. (1999) Protein Sci., 8, 800–809.
Feldman, H.J. and Hogue, C.W.V. (2000) Proteins Struct. Funct. Genet., 39, 112–131.
Fischer, M.W.F., Losonczi, J.A., Weaver, J.L. and Prestegard, J.H. (1999) Biochemistry, 38, 9013–9022.
Levitt, M. (1976) J. Mol. Biol., 104, 59–107.
Losonczi, J.A. and Prestegard, J.H. (1998) Biochemistry, 37, 706–716.
Losonczi, J.A., Andrec, M., Fischer, M.W.F. and Prestegard, J.H. (1999) J. Magn. Reson., 138, 334–342.
Markus, M.A., Gerstner, R.B., Draper, D.E. and Torchia, D.A. (1999) J. Mol. Biol., 292, 375–387.
Meiler, J., Blomberg, N., Nilges, M. and Griesinger, C. (2000) J. Biomol. NMR, 16, 245–252.
Mueller, G.A., Choy, W.-Y., Yang, D., Forman-Kay, J.D., Venters, R.A. and Kay, L.E. (2000) J. Mol. Biol., 300, 197–212.
Ottiger, M. and Bax, A. (1998) J. Am. Chem. Soc., 120, 12334–12341.
Quiocho, F.A., Spurlino, J.C. and Rodseth, L.E. (1997) Structure, 5, 997–1015.
Sharff, A.J., Rodseth, L.E., Spurlino, J.C. and Quiocho, F.A. (1992) Biochemistry, 31, 10657–10663.
Sharff, A.J., Rodseth, L.E. and Quiocho, F.A. (1993) Biochemistry, 32, 10553–10559.
Skrynnikov, N.R., Goto, N.K., Yang, D., Choy, W.-Y., Tolman, J.R., Mueller, G.A. and Kay, L.E. (2000) J. Mol. Biol., 295, 1265–1273.
Slichter, C.P. (1990) Principles of Magnetic Resonance, Springer-Verlag, New York, NY, pp. 605–614.
Tjandra, N., Grzesiek, S. and Bax, A. (1996) J. Am. Chem. Soc., 118, 6264–6272.
Tjandra, N. and Bax, A. (1997a) Science, 278, 1111–1114.
Tjandra, N. and Bax, A. (1997b) J. Magn. Reson., 124, 512–515.
Tjandra, N., Omichinski, J.G., Gronenborn, A.M., Clore, G.M. and Bax, A. (1997) Nat. Struct. Biol., 4, 732–738.
Tolman, J.R., Flanagan, J.M., Kennedy, M.A. and Prestegard, J.H. (1995) Proc. Natl. Acad. Sci. USA, 92, 9279–9283.
Tsui, V., Zhu, L.M., Huang, T.H., Wright, P.E. and Case, D.A. (2000) J. Biomol. NMR, 16, 9–21.
Wu, B., Arumugam, S., Gao, G.H., Lee, G., Semenchenko, V., Huang, W., Brew, K. and Van Doren, S.R. (2000) J. Mol. Biol., 295, 257–268.
Yang, D., Venters, R.A., Mueller, G.A., Choy, W.-Y. and Kay, L.E. (1999) J. Biomol. NMR, 14, 333–343.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Skrynnikov, N.R., Kay, L.E. Assessment of molecular structure using frame-independent orientational restraints derived from residual dipolar couplings. J Biomol NMR 18, 239–252 (2000). https://doi.org/10.1023/A:1026501101716
Issue Date:
DOI: https://doi.org/10.1023/A:1026501101716