Abstract
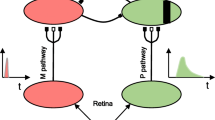
The aim of the study was to investigate spatiotemporal visual functions under scotopic and photopic conditions in order to acquire human psychophysical and electrophysiological data that are comparable with contrast sensitivities based on single-unit recordings in animal experiments. Static and dynamic contrast sensitivities (CSs) and steady-state visual evoked potentials (VEPs) were measured under photopic and scotopic conditions in healthy volunteers. The results from the CS experiment indicated that the inclusion of temporal modulation and the application of scotopic luminance levels uniformly resulted in a relatively increased sensitivity for low spatial frequencies. Similarly, analysis of the second harmonic component of the VEPs demonstrated a shift from band-pass to low-pass functions. These results suggest that, under scotopic conditions, human visuospatial processing is characteristically predominated by the functional activity of the magnocellular pathways.
Similar content being viewed by others
References
Lennie P. Parallel visual pathways: a review. Vision Res 1980; 20: 561–594.
Shapley R. Visual sensitivity and parallel retinocortical channels. Annu Rev Psychol 1990; 41: 635–658.
Bassi CJ, Lehmkuhle S. Clinical implications of parallel visual pathways. J Amer Opt Ass 1990; 61: 98–110.
Barnard N, Crewther SG, Crewther DP. Development of a magnocellular function in good and poor primary school-age readers. Optom Vis Sci 1998; 75: 62–68.
Demb JB, Boynton GM, Best M, Heeger DJ. Psychophysical evidence for a magnocellular pathway deficit in dyslexia. Vision Res 1998; 38: 1555–1559.
Steinman SB, Steinman BA, Garzia RP. Vision and attention. II: Is visual attention a mechanism through which a defi-cient magnocellular pathway might cause reading disability? Optom Vis Sci 1998; 75: 674–681.
Vogt U, Morland A, Migdal C, Ruddock K. Spatial and temporal visual filtering in patients with glaucoma and ocular hypertension. Eye 1998; 12: 691–696.
Vidyasagar TR, Pammer K. Impaired visual search in dyslexia relates to the role of the magnocellular pathway in attention. Neuroreport 1999; 10: 1283–1287.
Conte M, Zemon V, Camisa J, Milkman N. Comparing psychophysical and VEP contrast sensitivity. Invest Ophthalmol Vis Sci 1983; 24: S47.
Thompson DA, Drasdo N. Colour, contrast and the visual evoked potential. Opht Physiol Opt 1992; 12: 225–228.
Baseler HA, Bearse MA, Sutter EE. P/M channel ratio as a function of eccentricity revealed by a decomposition of the human VEP. Invest Ophthalmol Vis Sci 1995; 36: 942.
Klistorner A, Crewther DP, Crewther SG. Separate magnocellular and parvocellular contributions from temporal analysis of the multifocal VEP. Vision Res 1997; 37: 2161–2169.
Valberg A, Rudvin I. Possible contributions of magnocellularand parvocellular pathway cells to transient VEPs. Vis Neurosci 1997; 14: 1–11.
Kubova Z, Kuba M, Spekreijse H, Blakemore C. Contrast dependence of motion-onset and pattern-reversal evoked potentials. Vision Res 1995; 35: 197–205.
Tobimatsu S, Tomoda H, Kato M. Parvocellular and magnocellular contributions to visual evoked potentials in humans: stimulation with chromatic and achromatic gratings and apparent motion. J Neurol Sci 1995; 134: 73–82.
Regan D. Evoked potentials specific to spatial patterns of luminance and colour. Vision Res 1973; 13: 2381–2402.
Regan D. Human brain electrophysiology. New York: Elsevier, 1989.
Nakayama K, Mackeben M. Steady state visual evoked potentials in the alert primate. Vision Res 1982; 22: 1261–1271.
Kulikowski JJ. On the nature of visual evoked potentials, unit responses and psychophysics. In: Valberg A, Lee BB, eds. From pigments to perception, NATO ASI Series, New York: Plenum, 1991: 197–209.
Regan D, Lee BB. A comparison of the 40 Hz response in man and the properties of macaque ganglion cells. Vis Neurosci 1993; 10: 439–445.
Schultze M. Zur Anatomie und Physiologie der Retina. Arch Mikr Anat 1866; 2: 175.
Hassler R. Comparative anatomy of the central visual systems in day-and night-active primates. In: Hassler R, Stephan H. eds. Evolution of the forebrain, Stuttgart: Thieme Verlag, 1966.
Purpura K, Kaplan E, Shapley RM. Background light and the contrast gain of primate P and M retinal ganglion cells. Proc Natl Acad Sci USA 1988; 85: 4534–4537.
Kremers J, Lee BB, Kaiser PK. Sensitivity of macaque retinal ganglion cell and human observers to combined luminance and chromatic temporal modulation. J Opt Soc Am A 1992; 9: 1478–1485.
Lee BB, Smith VC, Pokorny J, Kremers J. Rod inputs to macaque ganglion cells. Vision Res 1997; 37: 2813–2828.
Enroth-Cugell C, Hertz BG, Lennie P. Convergence of rod and cone signals in the cat's retina. J Physiol (Lond) 1997; 269: 297–318.
Gouras P, Link K. Rod and cone interaction in dark-adapted monkey ganglion cells. J Physiol (Lond) 1966; 184: 499–510.
Lennie P, Fairchild MD. Ganglion cell pathways for rod vision. Vision Res 1994; 34: 477–482.
Drum B, Armaly MF, Huppert W. Scotopic sensitivity loss in glaucoma. Arch Ophthalmol 1986; 104: 712–717.
Glovinsky Y, Quigley HA, Drum B, Bissett RA, Jampel HD. A whole-field scotopic retinal sensitivity test for the detection of early glaucoma damage. Arch Opthalmol 1992; 110: 486–490.
Bodis-Wollner I, Onofrj MC, Marx MS, Mylin LH. Visual evoked potentials in Parkinson's disease: spatial frequency, temporal rate, contrast and the effect of dopaminergic drugs. In: Cracco RQ, Bodis-Wollner I, eds. Evoked potentials, New York: Alan R. Liss, 1986: 307–319.
Tweel LH. van der, Spekreijse H. Psychophysics and electrophysiology of a rod-achromat. Doc. Ophthalmol. Proc Ser Xth ISCERG Symposium, Los Angeles 1972 Dr. W. Junk BV. Publishers, The Hague, 1973 163–173.
Benedek G, Janáky M, Adamkovich N, Rubicsek G, Sáry G. Scotopic pattern-reversal visual evoked potentials. Clin Vision Sci 1993; 8: 47–54.
Robson JG. Spatial and temproal contrast sensitivity of the visual system. J Opt Soc Am A 1966; 56: 1141–1142.
Longhurst RS. Geometrical and physical optics, 3rd ed. London. Longman, 1990: 445–58.
Flamant F. Variation du diametre de la pupille de l'oeil en function de la brillance. Rev Opt 1948; 27: 751–758.
Patel AS. Spatial resolution by the human visual system. The effect of mean retinal illuminance. J Opt Soc Am A 1966; 56: 689–694.
Daitch JM, Green DG. Contrast sensitivity of the human peripheral retina. Vision Res 1969; 9: 947–952.
Kulikowski JJ. Some stimulus parameters affecting spatial and temporal resolution of human vision. Vision Res 1971; 1: 83–93.
Van Meeteren A, Vos JJ. Resolution and contrast sensitivity at low luminances. Vision Res 1972; 12: 825–833.
Kelly DH. Adaptation effects on spatio-temporal sine-wave thresholds. Vision Res 1972; 12: 89–101.
Koenderink JJ, Bouman MA, Bueno de Mesquita AE, Slappendel S. Perimetry of contrast detection thresholds of moving spatial sine wave patterns IV. The influence of the mean retinal illuminance. J Opt Soc Am 1978; 68: 860–865.
Hofmann MI, Barnes CS, Hallett PE. Detection of briefly flashed sine-gratings in dark-adapted vision. Vision Res 1990; 30: 1453–1466.
Derefeldt G, Lennerstand G, Lundt B. Age variations in normal human contrast sensitivity. Acta Ophthalmol (Copenhagen) 1979; 57: 679–690.
Lundt BL, Lennerstand G, Derefeldt G. Central and peripheral normal contrast sensitivity for static and dynamic gratings. Acta Ophthalmol (Copenhagen) 1983; 61: 171–182.
Hicks TP, Lee BB, Vidyasagar TR. The responses of cells in macaque lateral geniculate nucleus to sinusoidal gratings. J Physiol (Lond) 1983; 337: 183–200.
Hawken MJ, Parker AJ. Contrast sensitivity and orientation sensitivity in lamina IV of the striate cortex of old world monkey. Exp Brain Res 1984; 54: 367–372.
Merigan WH, Maunsell JH. How parallel are the primate visual pathways? Annu Rev Neurosci 1993; 16: 369–402.
Ginsburg AP, Evans DW, Cannon MW, Owsley C, Mulvanny D. Large-sample norms for contrast sensitivity. Optom Physiol 1984; 61: 80–84.
Long GM, May PA. Dynamic visual acuity and contrast sensitivity for static and flickered gratings in a college sample. Optom Vis Sci 1992; 69: 915–922.
Grünert U. Anatomical evidence for rod input to the parvocellular pathway in the visual system of the primate. Eur J Neurosci 1997; 9: 617–621.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Benedek, G., Benedek, K., Kéri, S. et al. Human scotopic spatiotemporal sensitivity: a comparison of psychophysical and electrophysiological data. Doc Ophthalmol 106, 201–207 (2003). https://doi.org/10.1023/A:1022548013313
Issue Date:
DOI: https://doi.org/10.1023/A:1022548013313