Abstract
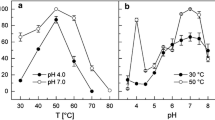
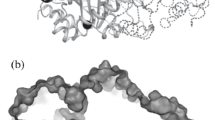
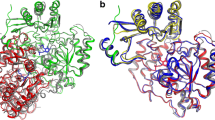
The crystal structure of pyrrolidone–carboxylate peptidase (PCP) from hyperthermophilic archaea Pyrococcus horikoshii (PhoPCP) has been determined at 1.6-A resolution by X-ray crystallography. PCP belongs to the C15 family of cysteine protease, and specifically removes the amino terminal pyroglutamate residue from a wide range of N-terminal-blocking peptides. The crystal structure is very similar to that of other hyperthermophiles, Pyrococcus friosus and Thermococcus litoralis, and even that from the mesophile, Bacillus amyloliquefacience. The inter-subunit disulfide bonds, which have been proposed as one of the thermostabilizing factors of the PCP from such hyperthermophiles, was not present in PhoPCP. The result suggests that the thermostability of PhoPCP may be obtained by the accumulation of many weak factors. Abbreviations: NCS – non-crystallographic symmetry; PCP – pyrrolidone-carboxylate peptidase; rmsd – root mean square deviation
Similar content being viewed by others
References
Doolittle, R.F., and Armentrout, R.W. (1968) Biochemistry 7, 516–521. medline+5644127
Fujiwara, K., Kobayashi, R., and Tsuru, D. (1979) Biochim. Biophys. Acta 570, 140–148. medline+39608
Uliana, J.A., and Doolittle, R.F. (1969) Arch. Biochem. Biophys. 131, 561–565. medline+5787224
Awade, A.C., Cleuziat, P., Gonzales, T., and Robert-Baudouy, J. (1994) Proteins. 20, 34–51. medline+7824521
Cummins, P.M., and O'Connor, B. (1998) Biochim. Biophys. Acta 1429, 1–17. medline+9920379
Folkers, K., Enzmann, F., Boler, J., Bowers, C.Y., and Schally, A.V. (1969) Biochem. Biophys. Res. Commun. 37, 123–126. medline+4981343
Nair, R.M., Barrett, J.F., Bowers, C.Y., and Schally, A.V. (1970) Biochemistry. 9, 1103–1106.
Matsuo, H., Baba, Y., Nair, R.M., Arimura, A., and Schally, A.V. (1971) Biochem. Biophys. Res. Commun. 43, 1334–1339.
Barelli, H., Vincent, J.P., and Checler, F. (1988) Eur. J. Biochem. 175, 481–489. medline+3409880
Carver, J.A., and Collins, J.G. (1990) Eur. J. Biochem. 187, 645–650. medline+2303058
Tanaka, H., Chinami, M., Mizushima, T., Ogasahara, K., Ota, M., Tsukihara, T., and Yutani, K. (2001) J. Biochem. 130, 107–118. PDB+1IOF, medline+11432786
Singleton, M., Isupov, M., and Littlechild, J. (1999) Structure Fold Des. 7, 237–244. PDB+1A2Z, medline+10368293
Singleton, M.R., and Littlechild, J.A. (2001) Methods Enzymol. 330, 394–403. medline+11210517
Odagaki, Y., Hayashi, A., Okada, K., Hirotsu, K., Kabashima, T., Ito, K., Yoshimoto, T., Tsuru, D., Sato, M., and Clardy, J. (1999) Structure Fold Des. 7, 399–411. PDB+1AUG, medline+
Ito, K., Inoue, T., Takahashi, T., Huang, H.S., Esumi, T., Hatakeyama, S., Tanaka, N., Nakamura, K.T., and Yoshimoto, T. (2001) J. Biol. Chem. 276, 18557–18562. medline+
Ogasahara, K., Khechinashvili, N.N., Nakamura, M., Yoshimoto, T., and Yutani, K. (2001) Eur. J. Biochem. 268, 3233–3242. medline+11389725
Kawarabayasi, Y., Sawada, M., Horikawa, H., Haikawa, Y., Hino, Y., Yamamoto, S., Sekine, M., Baba, S., Kosugi, H., Hosoyama, A., Nagai, Y., Sakai, M., Ogura, K., Otsuka, R., Nakazawa, H., Takamiya, M., Ohfuku, Y., Funahashi, T., Tanaka, T., Kudoh, Y., Yamazaki, J., Kushida, N., Oguchi, A., Aoki, K., and Kikuchi, H. (1998) DNA Res. 5, 55–76. medline+
Leslie, A.G.W. (1994) MOSFLM Users Guide, MRC-LMB, Cambridge, England.
Number 4 Collaborative Computional Project. (1994) Acta Crystallogr. D50, 760–763.
Jones, T.A., Zou, J.Y., Cowan, S.W., and Kjeldgaard. (1991) Acta Crystallogr. A47, 110–119. medline+2025413
Brunger, A.T., Adams, P.D., Clore, G.M., DeLano, W.L., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.S., Kuszewski, J., Nilges, M., Pannu, N.S., Read, R.J., Rice, L.M., Simonson, T., and Warren, G.L. (1998) Acta Crystallogr. D54, 905–921. medline+9757107
Genoscope. (http: //www.genoscope.cns.fr/pab/)
Le Saux, O., Gonzales, T., and Robert-Baudouy, J. (1996) J. Bacteriol. 178, 3308–3313. medline+8655512
Watanabe, K., Hata, Y., Kizaki, H., Katsube, Y., and Suzuki, Y. (1997) J. Mol. Biol. 269, 142–153. medline+9193006
Rawlings, N.D., and Barrett, A.J. (1994) Methods Enzymol. 244, 461–486. medline+7845226
Yoshimoto, T., Shimoda, T., Kitazono, A., Kabashima, T., Ito, K., and Tsuru, D. (1993) J. Biochem. 113, 67–73. medline+
Gonzales, T., Awade, A., Besson, C., and Robert-Baudouy, J. (1992) J. Chrom. 584, 101–107.
Kabashima, T., Li, Y., Kanada, N., Ito, K., and Yoshimoto, T. (2001) Biochim. Biophys. Acta 1547, 214–220. medline+
Laskowski, R.A., MacArthur, M.W., Moss, D.S., and Thornton, J.M. (1992) J. Appl. Crystallogr. 26, 283–291.
Singleton, M.R., Parratt, J.S., Taylor, S.J.C., and Littlechild, J.A. (2000) Extremophiles. 4, 297–303. medline+11057915
Tsunasawa, S., Nakura, S., Tanigawa, T., and Kato, I. (1998) J. Biochem. 124, 778–783. medline+9756623
Parkhill, J., Wren, B.W., Thomson, N.R., Titball, R.W., Holden, M.T., Prentice, M.B., Sebaihia, M., James, K.D., Churcher, C., Mungall, K.L., Baker, S., Basham, D., Bentley, S.D., Brooks, K., Cerdeno-Tarraga, A.M., Chillingworth, T., Cronin, A., Davies, R.M., Davis, P., Dougan, G., Feltwell, T., Hamlin, N., Holroyd, S., Jagels, K., Karlyshev, A.V., Leather, S., Moule, S., Oyston, P.C., Quail, M., Rutherford, K., Simmonds, M., Skelton, J., Stevens, K., Whitehead, S., and Barrell, B.G. (2001) Nature. 413, 523–527. medline+11586360
Gonzales, T., and Robert-Baudouy, J. (1994) J. Bacteriol. 176, 2569–2576. medline+7909543
Awade, A.C., Cleuziat, P., Gonzales, T., and Robert-Baudouy, J. (1992) FEBS Lett. 305, 67–73. medline+1353026
Cleuziat, P., Awade, A., and Robert-Baudouy, J. (1992) J. Mol. Microbiol. 6, 2051–2063. medline+1357525
Takami, H., Nakasone, K., Takaki, Y., Maeno, G., Sasaki, R., Masui, N., Fuji, F., Hirama, C., Nakamura, Y., Ogasawara, N., Kuhara, S., and Horikoshi, K. (2000) Nucleic Acids Res. 28, 4317–4331. medline+11058132
Daveran-Mingot, M.L., Campo, N., Ritzenthaler, P., and Le Bourgeois, P. (1998) J. Bacteriol. 180, 4834–4842. medline+
She, Q., Singh, R.K., Confalonieri, F., Zivanovic, Y., Allard, G., Awayez, M.J., Chan-Weiher, C.C., Clausen, I.G., Curtis, B.A., De Moors, A., Erauso, G., Fletcher, C., Gordon, P.M., Heikamp-de Jong, I., Jeffries, A.C., Kozera, C.J., Medina, N., Peng, X., Thi-Ngoc, H.P., Redder, P., Schenk, M.E., Theriault, C., Tolstrup, N., Charlebois, R.L., Doolittle, W.F., Duguet, M., Gaasterland, T., Garrett, R.A., Ragan, M.A., Sensen, C.W., and Van der Oost, J. (2001) Proc. Natl. Acad. Sci. USA 98, 7835–7840. medline+11427726
Patti, J.M., Schneider, A., Garza, N., and Boles, J.O. (1995) Gene 166, 95–99. medline+8529900
Vriend, G. (1990) J. Mol. Graph. 8, 52–56.
Kraulis, P.J. (1991) J. Appl. Crystallogr. 24, 946–950.
Merrit, E.A., and Murphy, M.E.P. (1994) Acta Crystallogr. D50, 869–873.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sokabe, M., Kawamura, T., Sakai, N. et al. The X-ray crystal structure of pyrrolidone–carboxylate peptidase from hyperthermophilic archaea Pyrococcus horikoshii. J Struct Func Genom 2, 145–154 (2002). https://doi.org/10.1023/A:1021257701676
Issue Date:
DOI: https://doi.org/10.1023/A:1021257701676