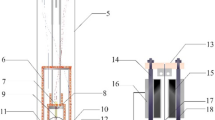
Abstract
An apparatus for the simultaneous measurement of viscosity and density of fluids is presented. The viscometer-densimeter covers a viscosity range up to 150 µPa⋅s and a density range up to 2000 kg⋅m−3 at temperatures from 233 to 523 K and pressures up to 30 MPa. Very accurate density measurements with uncertainties of ±0.02 to ±0.05% have always been carried out with this apparatus, although in its first version it was necessary to calibrate the viscosity measuring system on a reference fluid in order to achieve uncertainties of ±0.6 to ±1.0% in viscosity. After significant improvements, the apparatus now achieves uncertainties in viscosity of less than ±0.15% in the dilute gas region and less than ±0.4% for higher densities. Moreover, the viscosity measuring system can be described in an absolute way; calibration is no longer necessary. In order to test the advanced apparatus and to determine viscosity-density values of very high quality, comprehensive measurements on nitrogen, argon, and methane were carried out in the entire working range of the viscometer-densimeter. In addition, viscosity-density measurements on helium, neon, and krypton were made on two selected isotherms each. All measurements show that the estimated total uncertainty of ±0.15 to ±0.4% in viscosity and of ±0.02 to ±0.05% in density is clearly met. In order to verify the results of the combined viscometer-densimeter, a new apparatus for very accurate viscosity measurements was designed. While the working range of this apparatus is restricted to the dilute gas region, it yields uncertainties of less than ±0.07% in viscosity. Measurements carried out with this apparatus confirmed the previously measured values of the combined viscometer-densimeter within ±0.03%.
Similar content being viewed by others
REFERENCES
A. H. Krall, J. C. Nieuwoudt, J. V. Sengers, and J. Kestin, Fluid Phase Equil. 36:207 (1987).
A. A. H. Padua, J. M. N. A. Fareleira, J. C. G. Calado, and W. A. Wakeham, Int. J. Thermophys. 17:781 (1998).
F. Audonnet and A. A. H. Padua, Fluid Phase Equil. 181:147 (2001).
A. Docter, H. W. Lösch, and W. Wagner, Fortschr.-Ber. VDI-Z., Ser. 3, No. 494 (1997).
A. Docter, H. W. Lösch, and W. Wagner, Int. J. Thermophys. 20:485 (1999).
H. W. Lösch, R. Kleinrahm, and W. Wagner, in Jahrbuch 1994 “Verfahrenstechnik und Chemieingenieurwesen”, VDI Gesellschaft Verfahrenstechnik und Chemieingenieurwesen (VDI-Verlag, Düsseldorf, 1994), pp. 117–137.
W. Wagner, K. Brachthäuser, R. Kleinrahm, and H. W. Lösch, Int. J. Thermophys. 16:399 (1995).
W. Wagner, R. Kleinrahm, H. W. Lösch, and R. T. Watson, in IUPAC Experimental Thermodynamic, Vol. VI: Measurements of the Thermodynamic Properties of Single Phases, A. R. H. Goodwin, K. N. Marsh, and W. A. Wakeham, eds. (Elsevier, Amsterdam, 2002), in press.
V. Vasanta Ram, Eine Lösung der zeitabhängigen Navier-Stokes-Gleichungen für ein konzentrisches Zylindersystem (Arbeitsbericht des Lehrstuhls für Strömungsmechanik, Ruhr-Universität Bochum, 1996).
K. Stephan, R. Krauss, and A. Laesecke, J. Phys. Chem. Ref. Data 16:993 (1987).
E. Vogel, Ber. Bunsenges. Phys. Chem. 88:997 (1984).
A. G. Clarke and E. B. Smith, J. Chem. Phys. 51:4156 (1969).
J. Kestin, S. T. Ro, and W. A. Wakeham, Ber. Bunsenges. Phys. Chem. 86:753 (1982).
J. Kestin, E. Paykoc, and J. V. Sengers, Physica 54:1 (1971).
J. H. B. Hoogland, H. R. van den Berg, and N. J. Trappeniers, Physica 134A:169 (1985).
J. A. Gracki, G. P. Flynn, and J. Ross, J. Chem. Phys. 51:3856 (1969).
G. P. Flynn, R. V. Hanks, N. A. Lemaire, and J. Ross, J. Chem. Phys. 38:154 (1963).
A. Michels and R. O. Gibson, Proc. Roy. Soc. A134:288 (1931).
B. Latto and M. W. Saunders, Can. J. Chem. Eng. 50:765 (1972).
D. E. Diller, Physica 119A:92 (1983).
B. A. Younglove and H. J. M. Hanley, J. Phys. Chem. Ref. Data 15:1323 (1986).
G. C. Maitland and B. E. Smith, J. Chem. Eng. Data 17:150 (1972).
J. Kestin, S. T. Ro, and W. A. Wakeham, J. Chem. Phys. 56:4119 (1972).
R. A. Dawe and E. B. Smith, J. Chem. Phys. 52:693 (1970).
A. G. Clarke and E. B. Smith, J. Chem. Phys. 48:3988 (1968).
J. Kestin and W. Leidenfrost, Physica 25:1033 (1959).
J. Wilhelm and E. Vogel, Int. J. Thermophys. 21:301 (2000).
A. Michels, A. Botzen, and W. Schuurmann, Physica 20:1141 (1954).
W. M. Haynes, Physica 67:440 (1973).
J. Kestin and J. W. Whitelaw, Physica 29:335 (1963).
D. G. Friend, J. F. Ely, and H. Ingham, J. Phys. Chem. Ref. Data 18:583 (1989).
Y. Abe, J. Kestin, H. E. Khalifa, and W. A. Wakeham, Physica 93A:155 (1978).
J. Kestin and S. T. Ro, Ber. Bunsenges. Phys. Chem. 80:619 (1976).
D. E. Diller, Physica 104A:417 (1980).
J. G. Giddings, T. F. Kao, and R. Kobayashi, J. Chem. Phys. 45:578 (1966).
L. T. Carmichael, V. Berry, and B. H. Sage, J. Chem. Eng. Data 10:57 (1965).
J. J. Hurly and M. R. Moldover, J. Res. Nat. Inst. Stand. Tech. 105:667 (2000).
R. DiPippo and J. Kestin, Proc. Fourth Symp. Thermophys. Props. (ASME, New York, 1968), p. 304.
V. A. Rabinovich, A. A. Vassermann, V. I. Nedostup, and L. S. Veksler, Thermophysical Properties of Neon, Argon, Krypton, and Xenon, National Standard Reference Data Service of the USSR (Hemisphere Publishing, New York, 1988).
J. Kestin and A. Nagashima, J. Chem. Phys. 40:3648 (1964).
H. R. van den Berg, Precisiemetingen aan de Viscositeitscoefficient van Krypton en de logarithmische Term in de Dichtheidsontwikkeling. Dissertation (Universiteit van Amsterdam, 1979).
N. J. Trappeniers, A. Botzen, J. van Ooosten, and H. R. van den Berg, Physica 31:945 (1965).
R. Span, E. W. Lemmon, R. T. Jacobsen, W. Wagner, and A. Yokozeki, J. Phys. Chem. Ref. Data 29:1361 (2000).
C. Tegeler, R. Span, and W. Wagner, J. Phys. Chem. Ref. Data 28:779 (1999).
R. D. McCarty and V. D. Arp, Adv. Cryog. Eng. 35:1465 (1990).
J. Juza and O. Šifner, Acta Tech. CSAV 1:1 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Evers, C., Lösch, H.W. & Wagner, W. An Absolute Viscometer-Densimeter and Measurements of the Viscosity of Nitrogen, Methane, Helium, Neon, Argon, and Krypton over a Wide Range of Density and Temperature. International Journal of Thermophysics 23, 1411–1439 (2002). https://doi.org/10.1023/A:1020784330515
Issue Date:
DOI: https://doi.org/10.1023/A:1020784330515