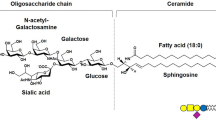
Abstract
Ganglioside GM3 was reported to induce the differentiation of HL-60 cells to differentiate along the macrophage-monocytic route. We used human monocytoid leukemia J6-2 cells and successfully induced differentiation by GM3. Because differentiation is accompanied by retarded growth rate and cell cycle is intimately related to phospholipid metabolism, so we explored how GM3 was related to phospholipid metabolism. By using [32P]Pi, [3H-CH3]choline, [3H-CH3]SAM, and [3H]inositol as radioactive tracers, we studied the turnover changes of phospholipids and their metabolites induced by GM3. For the morphological changes of differentiation to occur, the cells had to be treated with GM3 at a concentration of 50 μM for 5-6 days, but the phospholipid changes occurred at a very early stage of GM3 treatment (only 1 h). Our results indicate that GM3 stimulated PE methylation pathway inhibited both CDP-choline pathway and PI cycle. The phospholipid changes may constitute the early events in differentiation induced by GM3.
Similar content being viewed by others
REFERENCES (*Published in Chinese)
Hakomori, S. I. 2002. The glycosynapse. Proc. Natl. Acad. Sci. U.S.A. 99(1):225-232.
Hakomori, S. I. 2000. Traveling for the glycosphingolipid path. Glycoconj. J. 17:627-647.
Igarashi, Y., Nojiri, H., Hanai, N., and Hakomori, S. I. 1989. Gangliosides that modulate membrane protein function. Methods Enzymol. 179:521-541.
Hakomori, S. I. 2000. Cell adhesion/recognition and signal transduction through glycosphingolipid microdomains. Glycoconj. J. 17(3-4):143-151.
Pohlentz, G., Kaes, C., and Sandhoff, K. 1999. In vitro assays for enzymes of ganglioside synthesis. Methods Enzymol. 311:82-94.
Saito, M., Terui, Y., and Nojiri, H. 1985. An acidic glycosphingolipid, monosialo-ganglioside GM3, is a potent physiological inducer for monocytic differentiation of human promyelocytic leukemia cell line HL-60 cells. Biochem. Biophys. Res. Commun. 132(1):223-231.
Nojiri, H., Takaku, F., Terui, Y., Miura, Y., and Saito, M. 1986. Ganglioside GM3: An acidic membrane component that increases during macrophage-like cell differentiation can induce monocytic differentiation of human myeloid and monocytoid leukemic cell lines HL-60 and U937. Proc. Natl. Acad. Sci. U.S.A. 83(3):782-786.
Golfman, L. S., Bakovie, M., and Vance, D. E. 2001. Transcription of the CTP: Phosphocholine cytidylyltransferase alpha gene is enhanced during the S phase of the cell cycle. J. Biol. Chem. 276(47):43688-43692.
Khosokawa, M., Sato, A., Ishigamori, H., Kohno, H., Tanaka, T., and Takahashi, K. 2001. Synergistic effects of highly unsaturated fatty acid-containing phosphatidylethanolamine on differentiation of human leukemia HL-60 cells by dibutyryl cyclic adenosine monophosphate. Jpn. J. Cancer Res. 92(6):666-672.
*Wu, K. F., Zhang, Y. Q., Song, Y. H., and Feng, B. Z. 1986. Establishment and characterization of human leukemia cell lines (J6-1, J6-2, J6-3). Proc. Chinese Acad. Med. Sci. 1:218-222.
*Zhang, X., Cui, Z., Tang, N., and Zhu, Z. 1992. The differentiation of J6-2 cells induced by ganglioside GM3 and the comparison of glycosphingolipid and phospholipid composition before and after differentiation. Prog. Biochem. Biophys. (Beijing) 19(2):123-127.
Arai, K., Taki, T., Kondo, A., Matsumoto, M. 1988. Ganglioside GM3 as a modulator of differentiation of mouse myeloid leukemia cells (M1-T22). Cell Struct. Funct. 13(2): 161-169.
*Ma, K. L., Liu, Y., and Cui, Z. C. 1995. Ganglioside GM3 inhibits phosphatidylinositol turnover of human leukemia J6-2 cells. Prog. Biochem. Biophys. (Beijing) 22(3):249-253.
*Ma, K. L., Liu, Y., Wang, Z., and Cui, Z. C. 1996. The effect of ganglioside GM3 on the metabolism of phosphatidylcholine in human monocytoid leukemia J6-2 cells. Chinese Biochem. J. 12(3):365-369.
*Cui, Z. C., Zhang, X. B., Tang, N. M., and Zhu, Z. M. 1992. Effect of a cell differentiation inducer ganglioside GM3 on the phospholipid metabolism of a human monocytoid leukemia J6-2 cells. Chinese Biochem. J. 8(6):724-729.
Shields, D. J., Agellon, L. B., and Vance, D. E. 2001. Structure, expression profile and alternative processing of the human phosphatidylethanolamine N-methyltransferase (PEMT) gene. Biochim. Biophys. Acta 1532(1-2):105-114.
Pacheco, Y., Dubois, M., Prigent, A. F., Fonlupt, P., Timouyasse, L., Rey, C., Chambe, M. T., Biot, N., Perrin-Fayolle, M., and Pacheco, H. 1987. Phosphatidylethanolamine methyltransferase and cAMP, cGMP phosphodiesterases in lymphocytes and monocytes in sarcoidosis. Clin. Chim. Acta 163(3):267-277.
Davis, P. B. 1986. Lymphocyte and granulocyte phosphatidylethanolamine N-methyltransferase: Properties and activity in cystic fibrosis. Pediatr. Res. 20(12):1290-1296.
Cui, Z., Vance, J. E., Chen M. H., Voelker, D. R., and Vance, D. E. 1993. Cloning and expression of a novel phosphatidylethanolamine N-methyltransferase. A specific biochemical and cytological marker for a unique membrane fraction in rat liver. J. Biol. Chem. 268:16655-16663.
Cui, Z., Chen, Y. J., and Vance, D. E. 1997. Inverse correlation between expression of phosphatidylethanolamine N-methyltransferase-2 and growth rate of perinatal rat livers. Biochim. Biophys. Acta1346:10-16.
Zou, W., Li, Z. Y., Li, Y. L., Ma, K. L., and Cui, Z. C. 2002. Overexpression of PEMT2 downregulates the P13K/Akt signaling pathway in rat hepatoma cells. Biochim. Biophys. Acta (in press).
*Ma, K. L., Liu, Y., and Cui, Z. C. 1996. A simple and rapid micro-analytical method for phosphoinositides. Prog. Biochem. Biophys. (Beijing) 23(1):78-82.
Ferrell, I. E. and Huestis, W. H. 1984. Phosphoinositide metabolism and the morphology of human erythrocytes. J. Cell Biol. 98:1992-1998.
Vance, J. E., Pan, D. B., and Vance, D. E. Campenot, R. B. 1991. Biosynthesis of membrane lipids in rat axons. J. Cell Biol. 115:1061-1068.
*Zhang, X. B., Tang, N. M., Zhu, Z. M., and Cui, Z. C. 1991. The comparative study of protein kinase C activity in J6-2 leukemic cells before and after differentiation induced by ganglioside GM3. J. Dalian Med. Coll. 13(4):63-67.
Yorek, M., Jaipaul, N., Dunlap, J., and Bielefeldt, K. 1999. Endothelin-stimulated Ca2+ mobilization by 3T3-L1 adipocytes is suppressed by tumor necrosis factor-alpha. Arch. Biochem. Biophys. 361(2):241-251.
Masserini, M. and Ravasi, D. 2001. Role of sphingolipids in the biogenesis of membrane domains. Biochim. Biophys. Acta 1532: 149-161.
Maggio, B., Ariga, T., Calderon, R. O., and Yu, R. K. Ganglioside GD3 and GD3-lactone mediated regulation of the intermolecular organization in mixed monolayers with dipalmitoylphosphatidylcholine. Chem. Phys. Lipids 90(1-2):1-10.
Majewski, J., Kuhl, T. L., Kjaer, K., and Smith, G. S. 2001. Packing of ganglioside-phospholipid monolayers: An x-ray diffraction and reflectivity study. Biophys. J. 81(5): 2707-2715.
Yuan, C. and Johnston, L. J. 2001. Atomic force microscopy studies of ganglioside GM1 domains in phosphatidylcholine and phosphatidylcholine/cholesterol bilayers. Biophys. J. 81(2): 1059-1069.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cui, ZC., Ma, KL., Zhang, XB. et al. Effects of Ganglioside GM3 on Phospholipid Turnover of Human Leukemic J6-2 Cells. Neurochem Res 27, 771–778 (2002). https://doi.org/10.1023/A:1020200806352
Issue Date:
DOI: https://doi.org/10.1023/A:1020200806352