Abstract
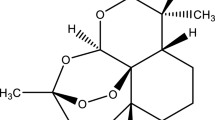
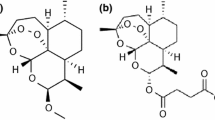
In simulated stomach acid (aqueous 0.01 M HC1, 37°C) β-arteether decomposed (half-life, 441 ± 17 min) to dihydroartemisinin, which subsequently rearranged to a new compound (1) having an endoperoxide group and an aldehyde group. The in vitro antimalarial activity of dihydroartemisinin is similar to that of β-arteether, whereas compound 1 had approximately 1/10th the activity of β-arteether. Compound 1 was prepared in sufficient quantities to afford samples for biological evaluation and a complete chemical characterization with 1H- and 13C-NMR and mass spectrometry. While β-arteether would be somewhat unstable in the stomach, if the drug were administered on an empty stomach (emptying time, ≈30 min) as a suspension or tablet, sufficient quantities of intact arteether may reach the small intestines, where it would be stable and readily absorbed. Its decomposition products, dihydroartemisinin and 1, may also contribute to the antimalarial activity of the administered drug following oral administration.
Similar content being viewed by others
REFERENCES
D. L. Klayman. Qinghaosu (artemisinin): An antimalarial drug from China. Science 228:1049–1055 (1985).
X. D. Luo and C. C. Shen. The chemistry, pharmacology, and clinical applications of Qinghaosu (artemisinin) and its derivatives. Med. Res. Rev. 7:29–52 (1987).
S. S. Zaman and R. P. Sharma. Some aspects of the chemistry and biological activity of artemisinin and related antimalarials. Heterocycles 32:1593–1638 (1991).
O. R. Idowu, J. L. Maggs, S. A. Ward, and G. Edwards. Decomposition reactions of arteether, a semisynthetic derivative of Qinghaosu (artemisinin). Tetrahedron 46:1871–1884 (1990).
A. Brossi, B. Venugopalan, L. d. Gerpe, H. J. C. Yeh, J. L. Flippen-Anderson, P. Buchs, X. D. Luo, W. Milhous, and W. Peters. Arteether, a new antimalarial drug: Synthesis and antimalarial properties. J. Med. Chem. 31:645–650 (1988).
F. S. El-Feraly, A. Ayalp, and M. A. Al-Yahya. Decomposition of dihydroartemistene on silica gel. J. Nat. Products 53:920–925 (1990).
J. K. Baker, H. N. ElSohly, and C. D. Hufford. Nuclear Overhauser effect spectroscopy (NOESY) and 3JHH coupling measurements in the determination of the conformation of the sesquiterpene antimalarial arteether in solution. Spectros. Lett. 23:111–122 (1990).
C. D. Hufford, I. S. Lee, H. N. ElSohly, H. T. Chi, and J. K. Baker. Structure elucidation and thermospray high-performance liquid chromatography/mass spectroscopy (HPLC/MS) of the microbial and mammalian metabolites of the antimalarial arteether. Pharm. Res. 7:923–927 (1990).
H. T. Chi and J. K. Baker. Use of deuterium-hydrogen exchange to characterize the fragmentation pathways of arteether and its metabolites in a thermospray mass spectrometer. Org. Mass. Spectrom., in press (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Baker, J.K., McChesney, J.D. & Chi, H.T. Decomposition of Arteether in Simulated Stomach Acid Yielding Compounds Retaining Antimalarial Activity. Pharm Res 10, 662–666 (1993). https://doi.org/10.1023/A:1018943329109
Issue Date:
DOI: https://doi.org/10.1023/A:1018943329109