Abstract
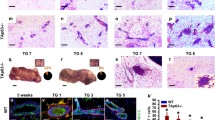
Post-lactational involution of the mammary glandprovides a system in which to study the expression andfunction of genes that regulate apoptosis in the contextof a normal tissue. The functions of the p53 tumor suppressor gene have been extensivelystudied as a mediator of apoptosis in response to DNAdamage, but its regulation in normal physiologicprocesses has been poorly characterized. Expression of p53 mRNA was shown to be among the firstgenes to be induced in mammary tissue following weaningof neonates. Although involution proceeds in the absenceof a functional p53 gene, it is delayed compared to normal individuals. Therefore, involutioncan be viewed as biphasic with initial responses beingsensitive to p53, whereas secondary responses beingp53-independent. These observations can be exploited to determine the subset of genes that arep53-responsive and that mediate the effects of p53 innormal mammary tissue.
Similar content being viewed by others
REFERENCES
M. T. Travers, M. C. Barber, E. Tonner, L. Quarrie, C. J. Wilde, and D. J. Flint (1996). The role of prolactin and growth hormone in regulation of casein gene expression and mammary cell survival: Relationships to milk synthesis and secretion. Endocrinology 137:1530–1539.
A. McTiernan and D. B. Thomas (1986). Evidence for a protective effect of lactation on risk of breast cancer in young women Am. J. Epidemiol. 124:353–358.
I. H. Russo and J. Russo (1993). Physiological bases of breast cancer prevention. Eur. J. Cancer Prevention 2(Suppl. 3): 101–111.
D. E. Henson and R. E. Tarone (1994). Involution and the etiology of breast cancer. Cancer 74:424–429.
D. L. Hadsell, N. M. Greenberg, J. M. Fligger, C. R. Baumrucker, and J. M. Rosen (1996). Targeted expression of des(1–3) human insulin-like growth factor I in transgenic mice influences mammary gland development and IGF-binding protein expression. Endocrinology 137:321–330.
R. Jager, U. Herzer, J. Schenkel, and H. Weiher (1997). Overexpression of Bcl-2 inhibits alveolar cell apoptosis during involution and accelerates c-myc-induced tumorigenesis of the mammary gland in transgenic mice. Oncogene 15:1787–1795.
E. P. Sandgren, J. A. Schroeder, T. H. Qui, R. D. Palmiter, R. L. Brinster, and D. C. Lee (1995). Inhibition of mammary gland involution is associated with transforming growth factor α but not c-myc-induced tumorigenesis in transgenic mice. Cancer Res. 55:3915–3927.
C. Coles, A. Condie, U. Chetty, C. M. Steel, H. J. Evans, and J. Prosser (1992). p53 mutations in breast cancer. Cancer Res. 52:5291–5298
M. S. Greenblatt, A. P. Grollman, and C. C. Harris (1996). Deletions and insertions in the p53 tumor suppressor gene in human cancers: Confirmation of the DNA polymerase slippage/misalignment model. Cancer Res. 56:2130–2136.
D. E. MacCallum, T. R. Hupp, C. A. Midgely, D. Stuart, S. J. Campbell, A. Harper, F. S. Walsh, E. G. Wright, A. Balmain, D. P. Lane, and P. A. Hall (1996) The p53 response to ionizing radiation in adult and developing murine tissues. Oncogene 13:2575–2587.
D. W. Meek (1998). Multisite phosphorylation and the integration of stress signals at p53. Cell Signal 10:159–166.
M. Serrano, A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe (1997). Oncogenic ras provokes premature cell senescence associated with accumulatin of p53 and p16INK4a. Cell 88:593–602.
S. Friend (1994). p53: A glimpse at the puppet behind the shadow play. Science 265:334–335.
K. K. Walker and A. J. Levine (1996). Identification of a novel p53 functional domain that is necessary for efficient growth suppression. Proc. Natl. Acad. Sci. U.S.A. 93:15335–15340.
E. M. Ruaro, L. Collavin, G. Del Sal, R. Haffner, M. Oren, A. J. Levine, and C. Schneider (1997). A proline-rich motif in p53 is required for transactivation-independent growth arrest as induced by Gasl. Proc. Natl. Acad. Sci. U.S.A. 94:4675–4680.
L. J. Ko and C. Prives (1996). p53: Puzzle and paradigm. Genes Dev. 10:1054–1072.
S. L. Madden, E. A. Galella, J. Zhu, A. H. Bertelsen, and G. A. Beaudry (1997). SAGE transcript profiles for p53–dependent growth regulation. Oncogene 15:1079–1085.
G. S. Wu, T. F. Burns, E. R. McDonald, W. Jiang, R. Meng, I. D. Krantz, G. Kao, D.-D. Gan, J.-Y. Zhou, R. Muschel, S. R. Hamilton, N. B. Spinner, S. Markowitz, G. Wu, and W. S. El-Deiry (1997). KILLER/DR5 is a DNA damage-inducible p53–regulated death receptor gene. Nat. Genetics 17:141–143.
C. Deng, P. Zhang, J. W. Harper, S. J. Elledge, and P. Leder (1995). Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82: 675–684.
T. Miyashita and J. C. Reed (1995). Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80:293–299.
K. Polyak, Y. Xia, J. L. Zweier, K. W. Kinzler, and B. Vogelstein (1997). A model for p53–induced apoptosis. Nature 389: 300–305.
Y. Shen and T. Shenk (1994). Relief of p53–mediated transcriptional repression by the adenovirus E1B 19–kDa protein or the cellular Bcl-2 protein. Proc. Natl. Acad. Sci. U.S.A. 91: 8940–8944.
S. Haldar, M. Negrini, M. Monne, S. Sabbioni, and C. M. Croce (1994). Down-regulation of bcl-2 by p53 in breast cancer cells. Cancer Res. 54:2095–2097.
T. Miyashita, M. Harigai, M. Hanada, and J. C. Reed (1994). Identification of a p53–dependent negative response element in the bcl-2 gene. Cancer Res. 54:3131–3135.
L. A. Donehower, M. Harvey, B. L. Slagle, M. J. McArthur, C. A. Montgomery, J. S. Butel, and A. Bradley (1992). Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumors. Nature 356:215–221.
V. P. Sah, L. D. Attardi, G. J. Mulligan, B. O. Williams, R. T. Bronson, and T. Jacks (1995). A subset of p53–deficient embryos exhibit exencephaly. Nat. Genetics 10:175–180.
J. F. Armstrong, M. H. Kaufman, D. J. Harrison, and A. R. Clarke (1995). High-frequency developmental abnormalities in p53–deficient mice. Curr. Biol. 5:931–936.
M. Colombel, F. Radvanyi, M. Blanche, C. Abbou, R. Buttyan, L. A. Donehower, D. Chopin, and J. P. Thiery (1994). Androgen suppressed apoptosis is modified in p53 deficient mice. Oncogene 10:1269–1274.
N. Almog and V. Rotter (1997). Involvement of p53 in cell differentiation and development. Biochim. Biophys. Acta 1333: F1–F27.
R. Strange, F. Li, S. Saurer, A. Burkhardt, and R. R. Friis (1992). Apoptotic cell death and tissue remodeling during mouse mammary gland involution. Development 115:49–58.
D. J. Jerry, C. Kuperwasser, S. R. Downing, J. Pinkas, C. He, E. S. Dickinson, S. Marconi, and S. P. Naber (1998). Delayed involution of the mammary epithelium in BALB/c-p53 nul1 mice. Oncogene 17:2305–2312.
B. Li, N. Greenberg, L. C. Stephens, R. Meyn, D. Medina, and J. M. Rosen (1994). Preferential overexpression of a 172Arg to Leu mutant p53 in the mammary gland of transgenic mice results in altered lobuloalveolar development. Cell Growth Differ. 5:711–721.
B. Li, F. S. Kittrell, D. Medina, and J. M. Rosen (1995). Delay of dimethylbenz[a]anthracene-induced mammary tumorigenesis in transgenic mice by apoptosis induced by an unusual mutant p53 protein. Mol. Carcinogen. 14:75–83.
M. Li, J. Hu, K. Heermeier, L. Hennighausen, and P. A. Furth (1996). Apoptosis and remodeling of mammary gland tissue during involution proceeds through p53–independent pathways. Cell Growth Differ. 7:13–20.
S. D. Bouffler, C. J. Kemp, A. Balmain, and R. Cox (1995). Spontaneous and ionizing radiation-induced chromosomal abnormalities in p53–deficient mice. Cancer Res. 55: 3883–3889.
S. Venkatachalam, Y.-P. Shi, S. N. Jones, H. Vogel, A. Bradley, D. Pinkel, and L. A. Donehower (1998). Retention of wild-type p53 in tumors from p53 heterozygous mice: Reduction of p53 dosage can promote cancer formation. EMBO J. 17:4657–4667.
K. Heermeier, M. Benedict, M. Li, P. Furth, G. Nunez, and L. Hennighausen (1996). Bax and Bcl-xs are induced at the onset of apoptosis in involuting mammary epithelial cells. Mech. Dev. 56:197–207.
R. L. Ludwig, S. Bates, K. H. Vousden, and L. Aagaard (1996). Differential activation of target cellular promoters by p53 mutants with impaired apoptotic function. Mol. Cell. Biol. 16:4952–4960.
E. Schneider, M. Montenarh, and P. Wagner (1998). Regulation of CAK kinase activity by p53. Oncogene 17:2733–2741.
L. R. Lund, J. Romer, N. Thomasset, H. Solberg, C. Pyke, M. J. Bissell, K. Dano, and Z. Werb (1996). Two distinct phases of apoptosis in mammary gland involution: Proteinase-independent and-dependent pathways. Development 122:181–193.
Z. Feng, A. Marti, B. Jehn, H. J. Altermatt, G. Chicaiza, and R. Jaggi (1995). Glucocorticoid and progesterone inhibit involution and programmed cell death in the mouse mammary gland. J. Cell Biol. 131:1095–1103.
M. Li, X. Liu, G. Robinson, U. Bar-Peled, K.-U. Wagner, W. S. Young, L. Hennighausen, and P. A. Furth (1997). Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proc. Natl. Acad. Sci. U.S.A. 94:3425–3430.
R. Jaggi, A. Marti, K. Guo, Z. Feng, and R. R. Friis (1996). Regulation of a physiological apoptosis: Mouse mammary involution. J. Dairy Sci. 79:1074–1084.
N. I. Walker, R. E. Bennett, and J. F. R. Kerr (1989) Cell death by apoptosis during involution of the lactating breast in mice and rats. Am. J. Anat. 185:19–32.
Rights and permissions
About this article
Cite this article
Jerry, D.J., Pinkas, J., Kuperwasser, C. et al. Regulation of p53 and Its Targets During Involution of the Mammary Gland. J Mammary Gland Biol Neoplasia 4, 177–181 (1999). https://doi.org/10.1023/A:1018777224808
Issue Date:
DOI: https://doi.org/10.1023/A:1018777224808