Abstract
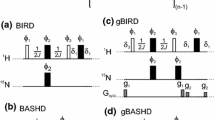
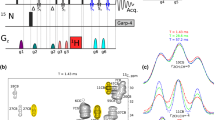
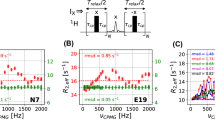
Many triple-resonance experiments make use of one-bond heteronuclear scalar couplings toestablish connectivities among backbone and/or side-chain nuclei. In medium-sized(15–30 kDa) proteins, short transverse relaxation times of Cα single-quantum stateslimit signal-to-noise (S/N) ratios. These relaxation properties can be improved usingheteronuclear multiple-quantum coherences (HMQCs) instead of heteronuclear single-quantumcoherences (HSQCs) in the pulse sequence design. In slowly tumbling macromolecules, theseHMQCs can exhibit significantly better transverse relaxation properties than HSQCs.However, HMQC-type experiments also exhibit resonance splittings due to multiple two- andthree-bond homo- and heteronuclear scalar couplings. We describe here a family of pulsed-field gradient (PFG) HMQC-type triple-resonance experiments using simultaneous 1H and13C constant-time (CT) periods to eliminate the t1 dependence of these scalar couplingeffects. These simultaneous CT PFG-(HA)CANH and PFG-(HA)CA(CO)NH HMQC-typeexperiments exhibit sharper resonance line widths and often have better S/N ratios than thecorresponding HSQC-type experiments. Results on proteins ranging in size from 6 to 30 kDashow average methine CαH HMQC:HSQC enhancement factors of 1.10 ± 0.15, withabout 40% of the cross peaks exhibiting better S/N ratios in the simultaneous CT-HMQCversions compared with the HSQC versions.
Similar content being viewed by others
References
Bax, A. and Grzesiek, S. (1993) Acc. Chem. Res., 26, 131–138.
Billeter, M., Neri, D., Otting, G., Qian, Y.Q. and Wüthrich, K. (1992) J. Biomol. NMR, 2, 257–274.
Boucher, W., Laue, E.D., Campbell-Burk, S. and Domaille, P.J. (1992a) J. Am. Chem. Soc., 114, 2262–2264.
Boucher, W., Laue, E.D., Campbell-Burk, S.L. and Domaille, P.J. (1992b) J. Biomol. NMR, 2, 631–637.
Clowes, R.T., Boucher, W., Hardman, C.H., Domaille, P.J. and Laue, E.D. (1993) J. Biomol. NMR, 3, 349–354.
Clubb, R.T. and Wagner, G. (1992) J. Biomol. NMR, 2, 389–394.
Clubb, R.T., Thanabal, V. and Wagner, G. (1992a) J. Magn. Reson., {vn97}, 213–217.
Clubb, R.T., Thanabal, V. and Wagner, G. (1992b) J. Biomol. NMR, {vn2}, 203–210.
Ernst, R.R., Bodenhausen, G. and Wokaun, A. (1987) Principles of NMR in One and Two Dimensions, Clarendon Press, Oxford, U.K.
Farmer II, B.T. and Venters, R.A. (1995) J. Am. Chem. Soc., 117, 4187–4188.
Feng, W., Rios, C.B. and Montelione, G.T. (1996) J. Biomol. NMR, {vn8}, 98–104.
Grzesiek, S. and Bax, A. (1992a) J. Am. Chem. Soc., 114, 6291–6293.
Grzesiek, S. and Bax, A. (1992b) J. Magn. Reson., 99, 201–207.
Grzesiek, S. and Bax, A. (1993) J. Biomol. NMR, 3, 185–204.
Grzesiek, S., Anglister, J. and Bax, A. (1993a) J. Magn. Reson., B101, 114–119.
Grzesiek, S., Anglister, J., Ren, H. and Bax, A. (1993b) J. Am. Chem. Soc., 115, 4369–4370.
Grzesiek, S. and Bax, A. (1995) J. Biomol. NMR, 6, 335–339.
Grzesiek, S., Kuboniwa, H., Hinck, A.P. and Bax, A. (1995) J. Am. Chem. Soc., 117, 5312–5315.
Ikura, M., Kay, L.E. and Bax, A. (1990) Biochemistry, 29, 4659–4667.
Kay, L.E., Ikura, M., Tschudin, R. and Bax, A. (1990) J. Magn. Reson., 89, 496–514.
Kay, L.E., Ikura, M. and Bax, A. (1991) J. Magn. Reson., 91, 84–92.
Kay, L.E., Keifer, P. and Saarinen, T. (1992) J. Am. Chem. Soc., 114, 10663–10665.
Kuboniwa, H., Grzesiek, S., Delaglio, F. and Bax, A. (1994) J. Biomol. NMR, 4, 871–878.
Logan, T.M., Olejniczak, E.T., Xu, R.X. and Fesik, S.W. (1992) FEBS Lett., 314, 413–418.
Lyons, B.A. and Montelione, G.T. (1993) J. Magn. Reson., B101, 206–209.
Lyons, B.A., Tashiro, M., Cedergren, L., Nilsson, B. and Montelione, G.T. (1993) Biochemistry, 32, 7839–7845.
Marion, D., Ikura, M., Tschudin, R. and Bax, A. (1989) J. Magn. Reson., 84, 393–399.
Montelione, G.T. and Wagner, G. (1989) J. Am. Chem. Soc., 111, 5474–5475.
Montelione, G.T. and Wagner, G. (1990) J. Magn. Reson., 87, 183–188.
Montelione, G.T., Lyons, B.A., Emerson, S.D. and Tashiro, M. (1992) J. Am. Chem. Soc., 114, 10974–10975.
Norwood, T.J. (1992) Prog. NMR Spectrosc., 24, 295–375.
Olejniczak, E.T., Xu, R.X., Petros, A.M. and Fesik, S.W. (1992) J. Magn. Reson., 100, 444–450.
Qian, X.-Y., Chien, C.-Y., Lu, Y., Montelione, G.T. and Krug, R.M. (1995) RNA, 1, 948–956.
Seip, S., Balbach, J. and Kessler, H. (1992) J. Magn. Reson., 100, 406–410.
Shaka, A.J., Barker, P.B. and Freeman, R. (1985) J. Magn. Reson., {vn64}, 547–552.
Wittekind, M. and Mueller, L. (1993) J. Magn. Reson., B101, 201–205.
Wüthrich, K. (1986) NMR of Proteins and Nucleic Acids, Wiley, New York, NY, U.S.A.
Yamazaki, T., Lee, W., Revington, M., Mattiello, D.L., Dahlquist, F.W., Arrowsmith, C.H. and Kay, L.E. (1994) J. Am. Chem. Soc., {vn116}, 6464–6465.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Swapna, G., Rios, C.B., Shang, Z. et al. Application of multiple-quantum line narrowing with simultaneous 1H and 13C constant-time scalar-coupling evolution in PFG-HACANH and PFG-HACA(CO)NH triple-resonance experiments. J Biomol NMR 9, 105–111 (1997). https://doi.org/10.1023/A:1018683920602
Issue Date:
DOI: https://doi.org/10.1023/A:1018683920602