Abstract
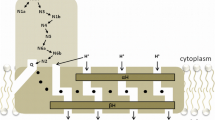
Ubiquinone is inhomogenously distributed in subcellular biomembranes. Apart from mitochondria where ubiquinone was demonstrated to exert bioenergetic and pathophysiological functions, unusually high levels of ubiquinone were also reported to exist in Golgi vesicles and lysosomes. In lysosomes the interior differs from other organelles by the low pH-value, which is important not only to arrest proteins but also to ensure optimal activity of hydrolytic enzymes. Since redox-cycling of ubiquinone is associated with the acceptance and release of protons, we assumed that ubiquinone is a part of a redox chain contributing to unilateral proton distribution. A similar function of ubiquinone was earlier suggested by Crane to operate in Golgivesicles. Support for the involvement of ubiquinone in a presumed couple of redox-carriers came from our observation that almost 70% of total lysosomal ubiquinone was in the divalently reduced state. Further reduction was seen in the presence of external NADH. Analysis of the components involved in the transfer of reducing equivalents from cytosolic NADH to ubiquinone revealed the existence of a FAD-containing NADH-dehydrogenase. The latter was found to reduce ubiquinone by means of a b-type cytochrome. Proton translocation into the interior was linked to the activity of the novel lysosomal redox chain. Oxygen was found to be the terminal electron acceptor, thereby also regulating acidification of the lysosomal matrix. In contrast to mitochondrial respiration, oxygen was only trivalently reduced, giving rise to the release of HO•-radicals. The role of this novel proton-pumping redox chain and the significance of the associated ROS formation has to be elucidated.
Similar content being viewed by others
References
Arai K, Kanaseki T and Ohkuma S (1991) Isolation of highly puri-fied lysosomes from rat liver: identification of electron carrier components on lysosomal membranes. J Biochem 110: 541-547
de Vries S, Albracht SP, Berden JA and Slater EC (1981) A new species of bound ubisemiquinone anion in QH2: cytochrome c oxidoreductase. J Biol Chem 256: 11996-11998
Echtay KS, Winkler E and Klingenberg M(2000) Coenzyme Q is an obligatory cofactor for uncoupling protein function. Nature 408: 609-613
Ford T, Graham J and Rickwood D (1994) Iodixanol: A nonionic iso-osmotic centrifugation medium for the formation of selfgenerated gradients. Anal Biochem 220: 360-366
Gille L and Nohl H (2000) The existence of a lysosomal redox chain and the role of ubiquinone. Arch Biochem Biophys 375: 347-354
Graham J, Ford T and Rickwood D (1994) The preparation of subcellular organelles from mouse liver in self-generated gradients of iodixanol. Anal Biochem 220: 367-373
Kalen A, Norling B, Appelkvist EL and Dallner G (1987) Ubiquinone biosynthesis by the microsomal fraction from rat liver. Biochim Biophys Acta 926: 70-78
Kozlov AV, Nohl H and Gille L (1998) Are reduced ubiquinones oxygen radical generators? Bioorg Chem 26: 334-344
Lang J, Gohil K and Packer L (1986) Simultaneous determination of tocopherols, ubiquinols and ubiquinones in blood, plasma, tissue homogenates and subcellular fractions. Anal Biochem 1567: 106-116
Lu AY, West SB, Ryan D and Levin W (1973) Characterization of partially purified cytochromes P-450 and P-448 from rat liver microsomes. Drug Metabolism and Disposition 1: 29-39
Mehlhorn RJ, Packer L, Macey R, Balaban AT and Dragutan I (1986) Measurement of transmembrane proton movements with nitroxide spin probes. Methods Enzymol 127: 738-745
Ohnishi T and King TE (1978) EPR and other properties of succinate dehydrogenase. Methods Enzymol 53: 483-495
Ohnishi T and Trumpower BL (1980) Differential effects of antimycin on ubisemiquinone bound in different environments in isolated succinate:cytochrome c reductase complex. J Biol Chem 255: 3278-3284
Staniek K and Nohl H (2000) Are mitochondria a permanent source of reactive oxygen species? Biochim Biophys Acta 1460: 268-275
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nohl, H., Gille, L. The bifunctional activity of ubiquinone in lysosomal membranes. Biogerontology 3, 125–131 (2002). https://doi.org/10.1023/A:1015288220217
Issue Date:
DOI: https://doi.org/10.1023/A:1015288220217