Abstract
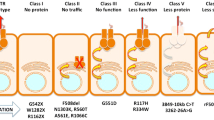
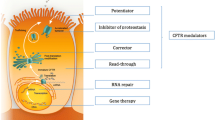
The disease Cystic Fibrosis (CF) is caused by mutations in the protein called CFTR, cystic fibrosis transmembrane conductance regulator, an ABC-transporter–like protein found in the plasma membrane of animal cells. CFTR is believed to function primarily as a Cl− channel, but evidence is mounting that this protein has other roles as well. Structurally, CFTR consists of a single polypeptide chain (1480 amino acids) that folds into 5 distinct domains. These include 2 transmembrane domains that are involved in channel formation; 2 nucleotide-binding domains (NBF1 and NBF2), the first of which clearly binds and hydrolyzes ATP; and 1 regulatory domain (R) that is phosphorylated in a cAMP-dependent process. Currently, the 3D structure of neither CFTR nor its domains has been elucidated, although both nucleotide domains have been modeled in 3D, and solution structures in 3D have been obtained for peptide segments of NBF1. The most common mutation causing CF is the deletion (Δ) of a single phenylalanine (F) in position 508 within a putative helix located in NBF1. CF patients bearing this ΔF508 mutation frequently experience chronic lung infections, particularly by Pseudomonas aeruginosa, and have a life span that rarely exceeds the age of 30. Since the CFTR gene was cloned and sequenced in 1989, there has been over a decade of research focused on understanding the molecular basis of CF caused by the ΔF508 mutation, with the ultimate objective of using the knowledge gained to carry out additional research designed to correct the underlying defect. In general, this pioneering or “ground roots” research has succeeded according to plan. This brief review summarizes some of the highlights with a focus on those studies conducted in the authors' laboratory. For us, this research has been both exciting and rewarding mainly because the results obtained, despite very limited funding, have provided considerable insight, not only into the chemical, molecular, and pathogenic basis of CF, but have made it possible for us and others to now develop novel, chemically rational, and “cost effective” strategies to identify agents that correct the structural defect in the Δ F508 CFTR protein causing most cases of CF.
Similar content being viewed by others
REFERENCES
Abrahams, J. P., Leslie, A. G., Lutter, R., and Walker, J. E. (1994). Nature 370, 621–628.
Akabas, M. H., Cheung, M., and Guinamard, R. (1997). J. Bioenerg. Biomembr. 29, 453–463.
Akabas, M. H., Kaufmann, C., Cook, T. A., and Archdeacon, P. (1994). J. Biol. Chem. 269, 14865–14868.
Aleksandrov, L., Mengos, A., Chang, X., Aleksandrov, A., and Riordan, J. R. (2001). J. Biol. Chem. 276, 12918–12923.
Arispe, N., Rojas, E., Hartman, J., Sorscher, E. J., and Pollard, H. B. (1992). Proc. Natl. Acad. Sci. U.S.A. 89, 1539–1543.
Ausman, D. J. (2001). Modern Drug Discovery, American Chemical Society, Washington, DC, pp. 33–39.
Baukrowitz, T., Hwang, T. C., Nairn, A. C., and Gadsby, D. C. (1994). Neuron 12, 473–482.
Bear, C. E., Li, C., Galley, K., Wang, Y., Garami, E., and Ramjeesingh, M. (1997). J. Bioenerg. Biomembr. 29, 465–473.
Bianchet, M. A., Hullihen, J., Pedersen, P. L., and Amzel, L. M. (1998). Proc. Natl. Acad. Sci. U.S.A. 95, 11065–11070.
Bianchet, M. A., Ko, Y. H., Amzel, L. M., and Pedersen, P. L. (1997). J. Bioenerg. Biomembr. 29, 503–524.
Brown, C. R., Hong-Brown, L. Q., Biwersi, J., Verkman, A. S., and Welch, W. J. (1996). Cell Stress, Chapter 1, 117–125.
Carson, M. R., Travis, S. M., and Welsh, M. J. (1995). J. Biol. Chem. 270, 1711–1717.
Cheng, S. H., Gregory, R. J., Marshall, J., Paul, S., Souza, D. W., White, G. A., O'Riordan, C. R., and Smith, A.(1990). Cell 63, 827–834.
Cheng, S. H., Rich, D. P., Marshall, J., Gregory, R. J., Welsh, M. J., and Smith, A. E. (1991). Cell 66, 1027–1036.
Dalemans, W., Barbry, P., Champigny, G., Jallat, S., Dott, K., Dryer, D., Crystal, R. G., Pavirani, A., Lecocq, J. P., and Lazdunski, M. (1991). Nature 354, 526–528.
Denning, G. M., Anderson, M. P., Amara, J. F., Marshall, J., Smith, A. E., and Welsh, M. J. (1992). Nature 358, 761–764.
Denning, G. M., Ostedgaard, L. S., and Welsh, M. J. (1992). J. Cell. Biol. 118, 551–559.
Diederichs, K., Diez, J., Greller, G., Muller, C., Breed, J., Schnell, C., Vonrhein, C., Boos, W., and Welte, W. (2000). EMBO J. 19, 5951–5961.
Doige, C. A., and Ames, G. F. (1993). Annu. Rev. Microbiol. 47, 291–319.
Gadsby, D. C., and Nairn, A. C. (1994). Trends Biochem. Sci. 19, 513–518.
Gruis, D. B., and Price, E. M. (1997). Biochemistry 36, 7739–7745.
Gunderson, K. L., and Kopito, R. (1994). J. Biol. Chem. 269, 19349–19353.
Gunteski-Hamblin, A. M., Greeb, J., and Shull, G. E. (1988). J. Biol. Chem. 263, 15032–15040.
Hager, K. M., Mandala, S. M., Davenport, J. W., Speicher, D. W., Benz, E. J., Jr., and Slayman, C. W. (1996). Proc. Natl. Acad. Sci. U.S.A. 83, 7693–7697.
Higgins, C. F. (1992). Annu. Rev. Cell. Biol. 8, 67–113.
Howell, L. D., Borchardt, R., and Cohen, J. A. (2000). Biochem. Biophys. Res. Commun. 271, 518–525.
Hung, L. W., Wang, I. X., Nikaido, K., Liu, P. Q., Ames, G. F., and Kim, S. H. (1998). Nature 396, 703–707.
Hwang, T. C., Nagel, G., Nairn, A. C., and Gadsby, D. C. (1994). Proc. Natl. Acad. Sci. U.S.A. 91, 4698–4702.
Imundo, L., Barasch, J., Prince, A., and Al-Awqati, Q. (1995). Proc. Natl. Acad. Sci. U.S.A. 92, 3019–3023.
Kano, I., Nagai, F., Satoh, K., Ushiyama, K., Nakao, T., and Kano, K. (1989). FEBS Lett. 250, 91–98.
Karpowich, N., Martsinkevich, O., Miller, L., Yuan, Y.-R., Dai, P. L., MacVey, K., Thomas, P. J., and Hunt, J. F. (2001). Structure 9, 571–586.
Ko, Y. H., Delannoy, M., and Pedersen, P. L. (1997a). Biochemistry 36, 5053–5064.
Ko, Y. H., Delannoy, M., and Pedersen, P. L. (1997b). FEBS Lett. 405, 200–208.
Ko, Y. H., and Pedersen, P. L. (1995). J. Biol. Chem. 270, 22093–22096.
Ko, Y. H., Thomas, P. J., Delannoy, M. R., and Pedersen, P. L. (1993). J. Biol. Chem. 268, 24330–24338.
Korst, R. J., McElvaney, N. G., Chu, C. S., Rosenfeld, M. A., Mastrangeli, A., Hay, J., Brody, S. L., Eissa, N. T., Danel, C., Jaffe, H. A., and Crystal, R. G. (1995). Am. J. Crit. Care Med. 151, S75–S87.
Li, C., Ramjeesingh, M., Wang, W., Garami, E., Hewryk, M., Lee, D., Rommens, J. M., Galley, K., and Bear, C. E. (1996). J. Biol. Chem. 271, 28463–28468.
Lu, N. T., and Pedersen, P. L. (2000). Arch. Biochem. Biophys. 375, 7–20.
Lukacs, G. L., Mohamed, A., Kartner, N., Chang, X. B., Riordan, J. R., and Grinstein, S. (1994). EMBO J. 13, 6076–6086.
Ma, J., Zhao, J. K., Drumm, M. L., Xie, J., and Davis, P. B. (1997). J. Biol. Chem. 272, 28133–28141.
Maita, T., Onishi, H., Yajima, E., and Matsuda, G. (1987). J. Biochem. 102, 133–145.
Massiah, M. A., Ko, Y. H., Pedersen, P. L., and Mildvan, A. S. (1999). Biochemistry 38, 7453–7461.
Mercer, R. W., Biemesderfer, D., Bliss, D. P., Jr., Collins, J. H., and Forbush, B. (1993). J. Cell. Biol. 121, 579–586.
Pasyk, E. A., and Foskett, J. K. (1995). J. Biol. Chem. 270, 12347–12350.
Peng, S., Sommerfelt, M., Logan, J., Huang, Z., Jilling, T., Kirk, K., Hunter, E., and Sorscher, E. (1993). Protein Expr. Purif. 4, 95–100.
Pier, G. B., Grout, M., Zaidi, T. S., Olsen, J. C., Johnson, L. G., Yankaskas, J. R., and Goldberg, J. B. (1996). Science 271, 64–67.
Ramjeesingh, M., Li, C., Garami, E., Huan, L. J., Galley, K., Wang, Y., and Bear, C. E. (1999). Biochemistry 38, 1463–1468.
Ramjeesingh, M., Li, C., Garami, E., Huan, L. J., Hewryk, M., Wang, Y., Galley, K., and Bear, C. E. (1997). Biochem. J. 327, 17–21.
Randak, C., Neth, P., Auerswald, E. A., Eckerskron, C., Assfalg-Machleidt, I., and Machleidt, W. (1997). FEBS Lett. 410, 180–186.
Riordan, J. R., Rommens, J. M., Kerem, B., Alon, N., Rozmahel, R., Grzelczak, Z., Zielenski, J., Lok, S., Plavsic, N., Chou, J. L., Drumm, M. L., Iannuzzi, M. C., Collins, F. S., and Tsui, L.-C. (1989). Science 245, 1066–1073.
Rosenfeld, M. A., Ronald, G., and Crystal, R. G. (1993). Pathol. Biol. (Paris) 41, 677–680.
Sato, S., Ward, C. L., Krouse, M. E., Wine, J. J., and Kopito, R. R. (1996). J. Biol. Chem. 271, 635–638.
Schultz, B. D., Bridges, R. J., and Frizzel, R. A. (1996). J. Memb. Biol. 151, 63–75.
Schulz, G. E., Elzinga, M., Marx, F., and Schirmer, R. H. (1974). Nature 250, 120–123.
Smith, J. J., Travis, S. M., Greenberg, E. P., and Welsh, M. J. (1996). Cell 85, 229–236.
Story, R. M., and Steitz, T. A. (1992). Nature 355, 374–376.
Tabcharani, J. A., Chang, X. B., Riordan, J. R., and Hanrahan, J. W. (1991). Nature 352, 628–631.
Takeyasu, K., Tamkun, M. M., Siegel, N. R., and Fambrough, D. M. (1987). J. Biol. Chem. 262, 10733–10740.
Thomas, P. J., Ko, Y. H., and Pedersen, P. L. (1992). FEBS Lett. 312, 7–9.
Thomas, P. J., and Pedersen, P. L. (1993). J. Bioenerg. Biomembr. 25, 11–19.
Thomas, P. J., Qu, B. H., and Pedersen, P. L. (1995). Trends Biochem. Sci. 20, 456–459.
Thomas, P. J., Shenbagamurthi, P., Sondek, J., Hullihen, J. M., and Pedersen, P. L. (1992). J. Biol. Chem. 267, 5727–5730.
Thomas, P. J., Shenbagamurthi, P., Ysern, X., and Pedersen, P. L. (1991). Science 251, 555–557.
Tsukihara, T., Aoyama, H., Yamashita, E., Tomizaki, T., Yamaguchi, H., Shinzawa-Itol, K., Nakashima, R., Yaono, R., and Yoshikawa, S. (1995). Science 269, 1069–1074.
Ward, C. L., and Kopito, R. R. (1994). J. Biol. Chem. 269, 25710–25718.
Welsh, M. J., and Ramsey, B. W. (1998). Am. J. Respir. Crit. Care Med. 157, S148–S154.
Welsh, M. J., Tsui, L.-C., Boat, T. F., and Beaudet, A. L. (1995). In: The Metabolic Basis of Inherited Disease (Scrivers, C. R., Beaudet, A. L., Sly, W. S., and Valle, D., eds.), McGraw-Hill, New York, pp. 3799–3876.
Wilkinson, D. J., Mansoura, M. K., Watson, P. Y., Smit, L. S., Collins, F. S., and Dawson, D. C. (1996). J. Gen. Physiol. 107, 103–119.
Wilson, J. M. (1993). Nature 365, 691–692.
Xia, D., Yu, C.-A., Kim, H., Xia, J.-Z., Kachurin, A. M., Zhang, L., Yu, L., and Deisenhofer, J. (1997). Science 277, 60–66.
Yuan, Y.-R., Blecker, S., Martsinkevich, O., Millen, L., Thomas, P. J., and Hunt, J. F. (2001). J. Biol. Chem. 276, 32313–32321.
Zaber, J., Coutre, L. A., Gregory, R. J., Graham, S. M., Smith, A. E., and Welsh, M. J. (1993). Cell 75, 207–216.
Zahllen, D. T. (2000). Trends Genet. 16, 272–275.
Rights and permissions
About this article
Cite this article
Ko, Y.H., Pedersen, P.L. Cystic Fibrosis: A Brief Look at Some Highlights of a Decade of Research Focused on Elucidating and Correcting the Molecular Basis of the Disease. J Bioenerg Biomembr 33, 513–521 (2001). https://doi.org/10.1023/A:1012831322753
Issue Date:
DOI: https://doi.org/10.1023/A:1012831322753