Abstract
Purpose. This study was undertaken to characterize the pharmacokinetic profiles of rifapentine and its active metabolite, 25-desacetyl-rifapentine, in elderly men.
Methods. Fourteen healthy, nonsmoking male volunteers between the ages of 65 and 82 years received a single oral 600 mg dose of rifapentine. Plasma samples were collected at frequent intervals for up to 72 hours postdose. The control group consisted of 20 healthy, young (18−45 years) male volunteers from a previous, single-dose (600 mg) rifapentine pharmacokinetic study.
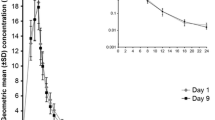
Results. Plasma rifapentine concentrations above the minimum inhibitory concentration for M. tuberculosiswere observed at 2 hours after dosing. Disposition of rifapentine was monophasic with a mean terminal half-life of 19.6 hours. The peak plasma concentration of 25-desacetyl-rifapentine was found 21.7 hours, on average, after the rifapentine dose; the mean 25-desacetyl-rifapentine t1/2was 22.9 hours. Compared to the younger subjects, apparent oral clearance of rifapentine (24%) was lower in the elderly male (p < 0.05), and Cmax (28%) was higher. The only adverse event reported in both the older and younger subjects in these single-dose studies was discoloration of the urine.
Conclusions. Because the age-related changes in the pharmacokinetic profile of rifapentine observed in this study were modest and unlikely to be associated with toxicity, no dosage adjustments for this antibiotic are recommended in elderly patients.
Similar content being viewed by others
REFERENCES
C. Truffot, R. Bismuth, and C. Boval. The in vitroand in vivoexperimental activity of cyclopentyl rifamycin (DL473) on Mycobacterium tuberculosis. Current Chemotherapy and Immunotherapy, Proceedings of the 12thInternational Congress of Chemotherapy. Florence, Italy, July 19–24, 1981. Abstract 693.
L. B. Heifets, P. J. Lindholm-Levy, and M. A. Flory. Bactericidal activity in vitroof various rifamycins against M. aviumand M. tuberculosis. Am. Rev. Respir. Dis. 141:626-630 (1990).
J. M. Dickinson and D. A. Mitchison. In vitro activity of selected rifamycins against rifampicin-resistant M. tuberculosisand MAIS-complex mycobacteria. Tubercle. 68:177-182 (1987).
W. Wehrli. Rifampin: mechanisms of action and resistance. Rev. Infect. Dis. 5(suppl 3):S407-S411 (1983).
F-J. Leinweber. Possible physiological roles of carboxylic ester hydrolases. Drug Metab. Rev. 18:379-439 (1987).
K. Reith, A. Keung, P. C. Toren, M. G. Eller, L. Cheng, and S. J. Weir. Mass balance and metabolism of 14C-rifapentine in healthy volunteers. Drug Metab. Dispos.(Accepted for publication).
A. C. F. Keung, T. D. Miller, V. I. Green, M. Ames, M. G. Eller, and S. J. Weir. Bioavailability (BA) and food effect study of rifapentine in healthy adults [Abstract S-8372]. Pharm. Res. 12(suppl):S419 (1995).
A. T. Birmingham, A. J. Coleman, M. L. E. Orme, B. K. Park, N. J. Pearson, A. H. Short, and P. J. Southgate. Antibacterial activity in serum and urine following oral administration in man of DL473 (a cyclopentyl derivative of rifampicin). Br. J. Clin. Pharmacol. 6:455P-456P (1978).
G. Buniva, D. Sassella, and G. M. Frigo. Pharmacokinetics of rifapentine in man. Proc. Int. Congr. Chemother. 111:29-33 (1983).
G. Acocella. Clinical pharmacokinetics of rifampicin. Clin. Pharmacokinet. 3:108-127 (1978).
S. Dawling and P. Crome. Clinical pharmacokinetic considerations in the elderly: an update. Clin. Pharmacokinet. 17:236-263 (1989).
W. A. Ritschel. Identification of populations at risk in drug testing and therapy: application to elderly patients. Eur. J. Drug Metab. Pharmacokinet. 18:101-111 (1993).
G. Tsujimoto, K. Hashimoto, and B. B. Hoffman. Pharmacokinetic and pharmacodynamic principles of drug therapy in old age. Part 1. Int. J. Clin. Pharmacol. Ther. Toxicol. 27:13-26 (1989).
K. W. Woodhouse. Pharmacokinetics of drugs in the elderly. J. Royal Soc. Med. 87(suppl 23):2-4 (1994).
M. A. Evans, E. J. Triggs, M. Cheung, G. A. Broe, and H. Creasey. Gastric emptying rate in the elderly: implications for drug therapy. J. Am. Geriatr. Soc. 29:201-205 (1981).
G. B. Forbes and J. C. Reina. Adult lean body mass declines with age: some longitudinal observations. Metabolism 19:653-663 (1970).
M. C. Geokas and B. J. Haverback. The ageing gastrointestinal tract. Am. J. Surg. 117:881-892 (1969).
L. P. Novak. Aging, total body potassium, fat-free mass, and cell mass in males and females between ages 18 and 85 years. J. Gerentol. 27:438-443 (1972).
C. Durnas, C-M. Loi, and B. J. Cusack. Hepatic drug metabolism and aging. Clin. Pharmacokinet. 19:359-389 (1990).
D. S. Chutka, J. M. Evans, K. C. Fleming, and K. G. Mikkelson. Drug prescribing for elderly patients. Mayo Clin. Proc. 70:685-693 (1995).
H. L. Rieder. Epidemiology of tuberculosis in Europe. Eur. Respir. J. 8(suppl 20):620s-632s (1995).
W. W. Stead and A. K. Dutt. Tuberculosis in the elderly. Semin. Respir. Infect. 4:189-197 (1989).
C. R. Horsburgh, Jr. Epidemiology of Mycobacterium aviumComplex disease. Am. J. Med. 102(5C):11-15 (1997).
F. Varga and E. Fischer. Age-dependent changes in blood supply of the liver and in the biliary excretion of eosin in rats. In: K. Kitani, ed. Liver and ageing. Elsevier-North, Amsterdam, 1978, pp. 327-340.
K. Kitani, S. Kanai, P. Miura, Y. Morita, and M. Kisahara. The effect of aging on the biliary excretion of ouabain in the rat. Exper. Gerontol. 13:9-17 (1978).
T. F. Blaschke and M. H. Skinner. The clinical pharmacokinetics of rifabutin. Clin. Infect. Dis. 22(suppl 1):S15-22 (1996).
A. Walubo, K. Chan, J. Woo, H. S. Chan. and C. L. Wong. The disposition of antituberculous drugs in plasma of elderly patients. II. Isoniazid, rifampicin and pyrazinamide. Meth. Find. Exp. Clin. Pharmacol. 13:551-556 (1991).
C. Advenier, C. Gobert, G. Houin, D. Bidet, S. Richelet, and J. P. Tillement. Pharmacokinetic studies of rifampicin in the elderly. Ther. Drug. Monitor. 5:61-65 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Keung, A.C.F., Eller, M.G. & Weir, S.J. Single-dose Pharmacokinetics of Rifapentine in Elderly Men. Pharm Res 15, 1286–1291 (1998). https://doi.org/10.1023/A:1011960428896
Issue Date:
DOI: https://doi.org/10.1023/A:1011960428896