Abstract
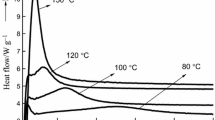
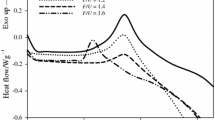
Differential scanning calorimetry(DSC) was used to study the effects of varying NaOH concentrations on the thermochemical curing properties of 2,4-dimethylol phenol (2,4-DMP), and 2,6-dimethylol phenol(2,6-DMP). Analysis of the DSC curves showed significant differences in the thermochemical curing behavior of these compounds with increasing NaOH:DMP molar ratios, in terms of the peak shape, position of the reaction peaks, (T p), along the temperature scale and energy of activation, E. The curves consisted of either a single, two or three exothermic peaks which indicated the occurrence of multiple reactions. One of these peaks was observed for the entire range of NaOH molar ratios, and is attributed to the self-condensation reaction. For the 2,4-DMP, NaOH had the effect of lowering the T p of curing from 212°C in the uncatalyzed state to135°C between 0.15–0.75 molar ratios. The lowest value of E, however, was 111 kJ mole−1, only through 0.45–0.60 molar ratios and this combined with the above, points to this concentration range as the optimum NaOH level. Similarly, the T p of curing for the 2,6-DMP was lowered from 211°C in the uncatalyzed state, to a minimum of 116°C at the NaOH:2,6-DMP molar ratio of 0.45. At this ratio, Ealso had the lowest value of 117 kJ mole−1 and this suggests that 0.45 molar ratio is the optimum NaOH level.
Similar content being viewed by others
References
Y. Yazaki and P. J. Collins, Adhesion Science and Technology, International Adhesion Symposium, Japan 1994, p. 607.
A. W. Christiansen and L. Gollob, J. Appl. Polym. Sci., 30 (1985) 2279.
A. Knop and W. Scheib, Chemistry and Application of Phenolic Resins, Springer-Verlag, 1979.
P. W. King, R. H. Mitchell and A. R. Westwood, J. Appl. Polym. Sci., 18 (1974) 1117.
K. C. Eapen and L. M. Yeddanapalli, Makromol. Chem., 119 (1968) 4.
A. Sebenik, I. Vizovisek and S. Lapanje, Eur. Polym. J., 10 (1974) 273.
G. R. Sprengling and J. H. Freeman, J. Am. Chem. Soc., 72 (1950) 1982.
J. H. Freeman and C. W. Lewis, J. Am. Chem. Soc., 76 (1954) 2080.
M. F. Grenier-Loustalot, S. Larroque, P. Grenier and D. Bedel, Polymer, 37 (1996) 939.
M. F. Grenier-Loustalot, S. Larroque and P. Grenier, Polymer, 35 (1994) 3046.
L. M. Yeddanapalli and D. J. Francis, Makromol. Chem., 55 (1962) 74.
D. J. Francis and L. M. Yeddanapalli, Makromol. Chem., 125 (1969) 119.
M. M. Sprung and M. T. Gladstone, J. Am. Chem. Soc., 71 (1949) 2907.
M. F. Grenier-Loustalot, S. Larroque and P. Grenier, Polymer, 37 (1996) 955.
S. So and A. Rudin, J. Appl. Polym. Sci., 41 (1990) 205.
G. Astarloa-Aierbe, J. M. Echeverria, J. L. Egiburu and I. Mondragon, Polymer, 40 (1999) 5873.
L. Y. Tonge, Y. Yazaki and A. S. Blicblau, J. Therm. Anal. Cal., 56 (1999) 1347.
J. H. Freeman, J. Am. Chem. Soc., 74 (1952) 6257.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tonge, L.Y., Hodgkin, J., Blicblau, A.S. et al. Effects of Initial Phenol-formaldehyde (PF) Reaction Products on the Curing Properties of PF Resin. Journal of Thermal Analysis and Calorimetry 64, 721–730 (2001). https://doi.org/10.1023/A:1011544411747
Issue Date:
DOI: https://doi.org/10.1023/A:1011544411747