Abstract
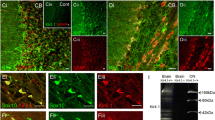
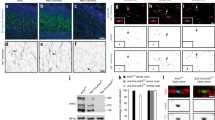
The localization of Shaker-type K+ channels in specialized domains of myelinated central nervous system axons was studied during development of the optic nerve. In adult rats Kv1.1, Kv1.2, Kv1.6, and the cytoplasmic β-subunit Kvβ2 were colocalized in juxtaparanodal zones. During development, clustering of K+ channels lagged behind that for nodal Na+ channels by about 5 days. In contrast to the PNS, K+ channels were initially expressed fully segregated from nodes and paranodes, the latter identified by immunofluorescence of Caspr, a component of axoglial junctions. Clusters of K+ channels were first detected at postnatal day 14 (P14) at a limited number of sites. Expression increased until all juxtaparanodes had immunoreactivity by P40. Developmental studies in hypomyelinating Shiverer mice revealed dramatically disrupted axoglial junctions, aberrant Na+ channel clusters, and little or no detectable clustering of K+ channels at all ages. These results suggest that in the optic nerve, compact myelin and normal axoglial junctions are essential for proper K+ channel clustering and localization.
Similar content being viewed by others
References
Bekele-Arcuri, Z., Matos, M. F. Manganas, M. M., Strassle, B. W., Monaghan, M. M., Rhodes, K. J. & Trimmer, J. S. (1996) Generation and characterization of subtype-specific monoclonal antibodies to K+ channel alpha and beta subunit polypeptides. Neuropharmacology 35, 851–865.
Bever, C. T. JR., Young, D., Anderson, P. A., Krumholz, A., Conway, K., Leslie, J., Eddington, N., Plaisance, K. I., Panitch, H. S. & Dhib-jalbut, S. (1994) The effects of 4-aminopyridine on multiple sclerosis patients: results of a randomized, placebo-controlled, double-blind, concentration-controlled, crossover trial. Neurology 44, 1054–1059.
Bostock, H., Sears, T. A. & Sherratt, R. M. (1981) The effects of 4-aminopyridine and tetraethylammonium ions on normal and demyelinated mammalian nerve fibres. Journal of Physiology 313, 301–315.
Chiu, S. Y. & Ritchie, J. M. (1980) Potassium channels in nodal and internodal axonal membrane of mammalian myelinated fibres. Nature 284, 170–171.
Chiu, S. Y. & Ritchie, J. M., (1981) Evidence for the presence of potassium channels in the paranodal region of acutely demyelinated mammalian single nerve fibres. Journal of Physiology 313, 415–437.
Chiu, S. Y. & Ritchie, J. M., Rogart, R. B., & Stagg, D. (1979) A quantitative description of membrane currents in rabbit myelinated nerve. Journal of Physiology 292, 149–166.
Dugandzija-Novakovic, S., Koszowski, A. G., Levinson, S. R. & Shrager, P. (1995) Clustering of Na channels and node of Ranvier formation in remyelinating axons. Journal of Neuroscience 15, 492–502.
Einheber, S., Zanazzi, G., Ching, W., Scherer, S., Milner, T. A., Peles, E. & Salzer, J. L. (1997) The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. Journal of Cell Biology 139, 1495–1506.
Fields, R. D. Black, J. A., Bowe, C. M., Kocsis, J. D. & Waxman, S. G. (1986) Differences in intramembranous particle distribution in the paranodal axolemma are not associated with functional differences of dorsal and ventral roots. Neuroscience Letters 67, 13–18.
Foster, R. E. Connors, B. W. & Waxman, S. G. (1982) Rat optic nerve: electrophysiological, pharmacological and anatomical studies during development. Brain Research 255, 371–386.
Gordon, T. R., Kocsis, J. D. & Waxman, S. G. (1988) Evidence for the presence of two types of potassium channels in the rat optic nerve. Brain Research 447, 1–9.
Gordon, T. R. Kocsis, J. D. & Waxman, S. G. (1989) Pharmacological sensitivities of two afterhyperpolarizations in rat optic nerve. Brain Research 502, 252–257.
Grissmer, S., Nguyen, A. N., Aiyar, J., Hanson, D. C., Mather, R. J., Gutman, G. A., Karmilowicz, M. J., Auperin, D. D. & Chandy, K. G. (1994) Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Molecular Pharmacology 45, 1227–1234.
Hildebrand, C. & Waxman, S. G. (1984) Postnatal differentiation of rat optic nerve fibers: electron microscopic observations on the development of nodes of Ranvier and axoglial relations. Journal of Comparative Neurology 224, 25–37.
Hopkins, W. F., Allen, M. L., Houamed, K. M. & Tempel, B. L. (1994) Properties of voltage-gated K+ currents expressed in Xenopus oocytes by mKv1.1, mKv1.2 and their heteromultimers as revealed by mutagenesis of the dendrotoxin-binding site in mKv1.1. Pflugers Archives 428, 382–390.
Hsueh, Y. P., Kim, E. & Sheng, M. (1997) Disulfidelinked head-to-head multimerization in the mechanism of ion channel clustering by PSD-95. Neuron 18, 803–814.
Huxley, Y. P. & Stampfli, R. (1949) Evidence for saltatory conduction in peripheral myelinated nerve fibres. Journal of Physiology 108, 315–339.
Inoue, Y., Nakamura, R., Mikoshiba, K. & Tsukada, Y. (1981) Fine structure of the central myelin sheath in the myelin deficient mutant Shiverer mouse, with special reference to the pattern of myelin formation by oligodendroglia. Brain Research 219, 85–94.
Isacoff, E. Y., Jan, Y. N. & Jan, L. Y. (1990) Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature 345, 530–534.
Kim, E., Niethammer, M., Rothschild, A., Jan, Y. N. & Sheng, M. (1995) Clustering of Shaker type K+ channels by interaction with a family of membrane associated guanylate kinases. Nature 378, 85–88.
Kim, E. & Sheng, M. (1996) Differential K+ channel clustering activity of PSD-95 and SAP97, two related membrane-associated putative guanylate kinases. Neuropharmacology 35, 993–1000.
Kim, E., Cho, K. O., Rothschild, A. & Sheng, M. (1996) Heteromultimerization and NMDA receptorclustering activity of Chapsyn-110, a member of the PSD-95 family of proteins. Neuron 17, 103–113.
Kocsis, J. D. & Waxman, S. G. (1980) Absence of potassium conductance in central myelinated axons. Nature 287, 348–349.
Kocsis, J. D. Waxman, S. G., Hildebrand, C. & Ruiz, J. A. (1982) Regenerating mammalian nerve fibres: changes in action potential waveform and firing characteristics following blockage of potassium conductance. Proceedings of the Royal Society of London, Series B 217, 77–87.
Laube, G., Roper, J., Pitt, J. C., Sewing, S., Kistner, U., Garner, C. C., Pongs, O. & Veh, R. W. (1996) Ultrastructural localization of Shaker-related potassium channel subunits and synapseassociated protein 90 to septate-like junctions in rat cerebellar Pinceaux. Brain Research Molecular Brain Research 42, 51–61.
Menegoz, M., Gaspar, P., Le Bert, M., Galvez, T., Burgaya, F., Palfrey, C., Ezan, P., Arnos, F. & Girault, (1997) Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron 19, 319–331.
Mi, H., Deerinck, T. J. Ellisman, M. H. & Schwarz, T. L. (1995) Differential distribution of closely related potassium channels in rat Schwann cells. Journal of Neuroscience 15, 3761–3774.
Nakahira, K., Shi, G., Rhodes, K. J. & Trimmer, J. S. (1996) Selective interaction of voltage-gated K+ channel beta-subunits with alpha-subunits. Journal of Biological Chemistry 271, 7084–7089.
Peles, E., Joho, K., Plowman, G. D. & Schlessinger, J. (1997) Close similarity between Drosophila neurexin IV and mammalian Caspr protein suggests a conserved mechanism for cellular interactions. Cell 88, 745–746.
Rasband, M. N., Trimmer, J. S., Schwarz, T. L., Levinson, S. R., Ellisman, M. H., Schachner, M. & Shrager, P. (1998) Potassium channel distribution, clustering, and function in remyelinating rat axons. Journal of Neuroscience 18, 36–47.
Rasband, M. N., Peles, E., Trimmer, J. S., Levinson, S. R., Lux, S. E. & Shrager, P. (1999) Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing central nervous system. Journal of Neuroscience 19, 7516–7528.
Rettig, J., Heinemann, S, H., Wunder, F., Lorra, C., Parcej, D. N., Dolly, J. O. & Pongs, O. (1994) Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature 369, 289–294.
Rhodes, K. J., Strassle, B. W., Monaghan, M. M., Bekelearcuri, Z., Matos, M. F. & Trimmer, J. S. (1997) Association and colocalization of the Kv-beta-1 and Kv-beta-2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes. Journal of Neuroscience 17, 8246–8258.
Ritchie, J. M. (1982) Sodium and potassium channels in regenerating and developing mammalian myelinated nerves. Proceedings of the Royal Society of London, Series B 215, 273–287.
Roach, A., Takahashi, N., Pravtcheva, D., Ruddle, F. & Hood, L. (1985) Chromosomal mapping of mouse myelin basic protein gene and structure and transcription of the partially deleted gene in shiverer mutant mice. Cell 42, 149–155.
Roper, J. & Schwarz, J. R. (1989) Heterogeneous distribution of fast and slow potassium channels in myelinated rat nerve fibres. Journal of Physiology (London) 416, 93–110.
Rosenbluth, J. (1980) Central myelin in the mouse mutant shiverer. Journal of Comparative Neurology 194, 639–648.
Rosenbluth, J. (1981) Axoglial junctions in the mouse mutant Shiverer. Brain Research 208, 283–297.
Rosenbluth, J. (1984) Membrane specialization at the nodes of ranvier and paranodal and juxtaparanodal regions of myelinated central and peripheral nerve fibers. In The Node of Ranvier (edited by Zagoren, J. C. & Fedoroff, S.), pp. 31–65. Orlando, FL: Academic.
Sherratt, R. M., Bostock, H. & Sears, T. A. (1980) Effects of 4-aminopyridine on normal and demyelinated mammalian nerve fibres. Nature 283, 570–572.
Shi, G., Nakahira, K., Hammond, S., Rhodes, K. J., Schechter, L. E. & Trimmer, J. S. (1996) Beta subunits promote K+ channel surface expression through effects early in biosynthesis. Neuron 16, 843–852.
Smart, S. L., Lopantsev, V., Zhang, C. L., Robbins, C. A., Wang, H., Chiu, S. Y., Schwartzkroin, P. A., Messing, A., Tempel, B. L. (1998) Deletion of the Kv1.1 potassium channel causes epilepsy in mice. Neuron 20, 809–819.
Stephens, G. J., Garratt, J. C., Robertson, B. & Owen, D. G. (1994) On the mechanism of 4-aminopyridine action on the cloned mouse brain potassium channel mKv1.1. Journal of Physiology 477 (Part 2), 187–196.
Stuhmer, W., Ruppersberg, J. P., Schroter, K. H. Sakmann, B., Stocker, M., Giese, K. P., Pershke, A., Baumann, A. & Pongs, O. (1989) Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO 8, 3235–3244.
Stys, P. K., Ransom, B. R. & Waxman, S. G. (1991) Compound action potential of nerve recorded by suction electrode: a theoretical and experimental analysis. Brain Research 546, 18–32.
Tejedor, F. J., Bokhari, A., Rogero, O., Gorczyca, M., Zhang, J., Kim, E., Sheng, M. & Budnik, V. (1997) Essential role for dlg in synaptic clustering of Shaker K+ channels in vivo. Journal of Neuroscience 17, 152–159.
Trimmer, J. S. (1998) Regulation of ion channel expression by cytoplasmic subunits. Current Opinions in Neurobiology 8, 370–374.
Vabnick, I., Novakovic, S. D., Levinson, S. R., Schachner, M. & Shrager, P. (1996) The clustering of axonal sodium channels during development of the peripheral nervous system. Journal of Neuroscience 16, 4914–4922.
Vabnick, I., Trimmer, J. S., Schwarz, T. L. Levinson, S. R. Risal, D. & Shrager, P. (1999) Dynamic potassium channel distributions during axonal development prevent aberrant firing patterns. Journal of Neuroscience 19, 747–758.
Wang, H., Kunkel, D. D., Martin, T. M., Schwartzkroin, P. A. & Tempel, B. L. (1993) Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature 365, 75–79.
Wang, H., Allen, M. L., Grigg, J. J., Noebels, J. L., & Tempel, B. L. (1995) Hypomyelination alters K+ channel expression in mouse mutants shiverer and Trembler. Neuron 15, 1337–1347.
Wiggins, R. C., Chongjie, G., Delaney, C. & Samorajski, T. (1988) Development of axonaloligodendroglial relationships and junctions during myelination of the optic nerve. International Journal of Developmental Neuroscience 6, 233–243.
Zhou, L., Zhang, C. L., Messing, A. & Chiu, S. Y (1998) Temperature-sensitive neuromuscular transmission in Kv1.1 null mice: role of potassium channels under the myelin sheath in young nerves. Journal of Neuroscience 18, 7200–7215.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rasband, M.N., Trimmer, J.S., Peles, E. et al. K+ channel distribution and clustering in developing and hypomyelinated axons of the optic nerve. J Neurocytol 28, 319–331 (1999). https://doi.org/10.1023/A:1007057512576
Issue Date:
DOI: https://doi.org/10.1023/A:1007057512576