Abstract
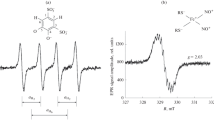
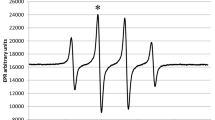
Nitric oxide (NO·) is a free radical characterized by a high spontaneous chemical reactivity with many other molecules including the superoxide radical (O2·−). This complex interaction may generate a peroxynitrite anion (ONOO−), which behaves as an important mediator of oxidative stress in many pathological states. In the present study, in vitro experiments were performed to assess directly the O2·− and hydroxyl (·OH) radical scavenging effects of various NO· donor drugs, i.e. sodium nitroprusside (SNP), sodium nitrite (NaNO2), molsidomine and SIN 1, at pH 7.4, 7 or 6. Concentrations of NO· in the incubation medium containing the different NO· donor drugs were measured by the assay based on the reaction of Fe-N-methyl-D-glucamine dithiocarbamate (MGD) with NO· that yields a stable spin-adduct measured by electron paramagnetic resonance (EPR). O2·− and ·OH generation was characterized by EPR spin trapping techniques, using the spin trap 5,5-dimethyl-1-pyrroline-1-oxide (DMPO). These free radicals were generated from the enzymatic system xanthine-xanthine oxidase, in phosphate buffer adjusted at pH 7.4, 7 and 6. Under these experimental conditions, SNP exhibited the strongest superoxide scavenging properties, characterized by IC50 values expressed in the µmolar range, which decreased at low pH. Addition of SNP (800 µM) to solution containing MGD and Fe2+ (5:1) at pH 7 4 produced a three line EPR spectrum which is identified to [(MGD)2-Fe2+-NO]. In control experiments no EPR signal was observed. We obtained the same results with NaNO2 and an augmentation of the spin-adduct level was noted with the prolongation of the incubation period. In return, molsidomine (2 mM) did not produce, in our conditions, a detectable production of NO·. NaNO2 displayed a significant superoxide scavenging effect only at pH 6, whilst neither molsidomine nor SIN 1 had any effect. Therefore, the superoxide scavenging properties of SNP, NaNO2, and molsidomine appeared to be closely related to their potential for NO· release, which partially depends on the pH conditions. The behaviour of SIN 1 is more complicated, the speed of oxygen diffusion probably acting as a limiting factor in NO· formation in our conditions. The production of NO· was detected in presence of SIN 1. The intensity of the complex is comparable with the signal founded with NaNO2. By contrast, all molecules exhibited hydroxyl radical scavenging properties, highlighting the capacity of ·OH to react with a wide range of molecules. In conclusion, considering the poor chemical reactivity of O2·−, the NO· donor drugs/O2·− interactions suggest a special relationship between these two radical species, which, in certain pathological states, could lead to the generation of cytotoxic end-products with strong oxidizing properties.
Similar content being viewed by others
References
Moncada S, Palmer RMJ, Higgs EA: Nitric oxide: Physiology, pathophysiology and pharmacology. Pharmacol Rev 43: 109–142, 1991
Gryglewski RJ, Palmer RMJ, Moncada S: Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320: 454–456, 1986
McCall TB, Boughton-Smith NK, Palmer RMJ, Whittle BJR, Moncada S: Synthesis of nitric oxide from L-arginine by neutrophils. Release and interaction with superoxide anion. Biochem J 261: 293–296, 1989
Cazevieille C, Müller A, Meynier F, Bonne C: Superoxide and nitric oxide cooperation in hypoxia/reoxygenation-induced neuron injury. Free Rad Biol Med 14: 389–395, 1993
Kubes P, Kanwar S, Niu X-F and Gaboury JP: Nitric oxide synthesis inhibition induces leukocyte adhesion via superoxide and mast cells. FASEB J 7: 1293–1299, 1993
Hogg N, Darley-Usmar VM, Wilson MT and Moncada S: The oxidation of a-tocopherol in human low-density lipoprotein by the simultaneous generation of superoxide and nitric oxide. FEBS Lett 326: 199–203, 1993
Kooy N, Royall JA, Ischiropoulos H, Beckman JS: Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Rad Biol Med 16: 149–156, 1994
Radi R, Rodriguez M, Castro L, Telleri R: Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Bioph 308: 89–95, 1994
Lafon-Cazal M, Culcasi M, Gaven F, Pietri S, Bockaert J: Nitric oxide, superoxide and peroxynitrite: Putative mediators of NMDA-induced cell death in cerebellar granule cells. Neuropharmacol 32: 1259–1266, 1993
Lipton SA, Chol Y-B, Pan Z-H, Lel SZ, Chen H-SV, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS: A redox-based mechanism for the neuro-protective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 364: 626–632, 1993
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA: Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Nat Acad Sci USA 87: 1620–1624, 1990
Moro MA, Darley-Usmar VM, Goodwin DA, Read NG, Zamora-Pino R, Feelisch M, Radomski MW, Moncada S: Paradoxical fate and biological action of peroxynitrite on human platelets. Proc Nat Acad Sci USA 91: 6702–6706, 1994
Siegfried MR, Erhardt J, Rider T, Ma X-L, Lefer AM: Cardioprotection and attenuation of endothelial dysfunction by organic nitric oxide donors in myocardial ischemia-reperfusion. J Pharmacol Exp Ther 260: 668–675, 1992
Foucher-Lavergne A, Kolsky H, Spreux-Varoquaux O, Delonca J, Beaufils P: Hemodynamics, tolerability and pharmacokinetics of linsidomine (SIN 1) infusion during the acute phase of uncomplicated myocardial infarction. J Cardiovasc Pharmacol 22: 779–784, 1993
Bohn H, Schönafinger K: Oxygen and oxidation promote the release of nitric oxide from sydnonimines. J Cardiovasc Pharmacol 14 (Suppl. 11): S6–S12, 1989
Varoquaux O, Cordonnier P, Dutot C: Pharmacokinetics of molsido-mine and SIN 1 in healthy volunteers, elderly patients and coronary patients. Eur J Clin Pharmacol 36 (Suppl.): A302, 1989
Feelisch M: The biochemical pathways of nitric oxide formation from nitrovasodilators: appropriate choice of exogenous NO· donors and aspects of preparation and handling of aqueous NO· solutions. J Cardiovasc Pharmacol 17 (Suppl. 3): S25–S33, 1991
Joseph J, Kalyanaraman B, Hyde JS: Trapping of nitric oxide by nitronyl nitroxides: an electron spin resonance investigation. Biochem Biophys Res Commun 192: 926–934, 1993
Johnson G, Tsao PS and Lefer A: Cardioprotective effects of authentic nitric oxide in myocardial ischemia with reperfusion. Crit Care Med 19: 244–252, 1991
Shirasaki Y, Su C: Endothelium removal augments vasodilation by sodium nitroprusside and sodium nitrite. Eur J Pharmacol 114: 93–96, 1985
Noack E, Feelisch M: Molecular aspects underlying the vasodilator action of molsidomine. J Cardiovasc Pharmacol 14 (Suppl. 11): S1–S5, 1989
Feelisch M, Ostrowski J, Noack E: On the mechanism of NO· release from sydnonimines. J Cardiovasc Pharmacol 14 (Suppl. 11): S13–S22, 1989
Shinobu LA, Jones SG, Jones MM: Sodium N-Methyl-D-Glucamine Dithiocarbamate and cadmium intoxication. Acta Pharmacol Toxicol 54: 189–194, 1984
Komarov A, Mattson D, Jones MM, Singh PK, Lai CS: In vivo spin trapping of nitric oxide in mice. Biochem Biophys Res Comm 195: 1191–1198, 1993
Obolenskaya MY, Vanin AF, Mordvintcev PI, Mulsch A, Decker K: EPR evidence of nitric oxide production by the regenerating rat liver. Bioch Biophys Res Comm 202: 571–576, 1994
Zweier JL, Wang P, Kuppusamy P: Direct measurement of nitric oxide generation in the ischemic heart using Electron Paramagnetic Resonance Spectroscopy. J Biol Chem 270: 304–307, 1995
Kruszyna H, Kruszyna R, Rochelle LG, Smith RP, Wilcox DE: Effects of temperature, oxygen, heme ligands and sulphydryl alkylation on the reactions of nitroprusside and nitroglycerine with hemoglobin. Biochem Pharmacol 46: 95–102, 1993
Ignarro LJ, Edwards JC, Gruetter DY, Barry BK, Gruetter CA: Possible involvement of S-nitrosothiols in the activation of guanylate cyclase by nitroso compounds. FEBS Lett 110: 275–278, 1980
Ignarro LJ, Lippton H, Edwards JC, Baricos WH, Hyman AL, Kadowitz PJ, Gruetter CA: Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther 218: 739–749, 1981
Kreye VAW: Direct vasodilators with unknown modes of action: the nitro-compounds and hydralazine. J Cardiovasc Pharmacol 6 (Suppl.4): S646–S655, 1984
Wilcox DE, Kruszyna H, Kruszyna R, Smith RP: Effect of cyanide on the reaction of nitroprusside with hemoglobin: Relevance to cyanide interference with the biological activity of nitroprusside. Chem Res Toxicol 3: 71–76, 1990
Kreye VAW: Sodium nitroprusside: approaches towards the elucida-tion of its mode of action. Trends In Pharmacol Sci 384–388, 1980
Garlick PB, Radda GK, Seeley PJ: Studies of acidosis in the ischaemic heart by phosphorus nuclear magnetic resonance. Biochem J 184: 547–554, 1979
Kruszyna H, Kruszyna R, Smith RP, Wilcox DE: Red blood cells generate nitric oxide from directly acting nitrogenous vasodilators. Toxicological Applied in Pharmacology 91: 429–438, 1987
Mülsch A, Vanin A, Mordvintcev P, Hauschildt S, Busse R: NO· accounts completely for the oxygenated nitrogen species generated by enzymatic L-arginine oxygenation. Biochem J 288: 597–603, 1992
Wang Y, Rochelle LG, Kruszyna H, Kruszyna R, Smith RP, Wilcox DE: An impurity present in some samples of SIN 1 oxidizes it to nitric oxide in anaerobic solutions. Toxicol 88: 165–176, 1994
Hogg N, Darley-Usmar VM, Wilson MT and Moncada S: Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem J 281: 419–424, 1992
Huie RE, Padmaja S: The reaction of NO· with superoxide. Free Rad Res Com 18: 195–199, 1993
Butler AR, Flitney FW, Williams DL: NO·, nitrosium ions, nitroxide ions, nitrosothiols and iron-nitrosyls in biology: A chemist's perspective. Trends In Pharmacol Sci 16: 18–22, 1995
Koppenol WH: The paradox of oxygen: Thermodynamics versus toxicity. Oxidases and Related Redox Systems, Alan R. Liss Inc., 1988, pp 93–109
Sawyer DT, Valentine JS: How super is superoxide? Academic Chemical Research 14: 393–400, 1981
Radi R, Beckman JS, Bush KM, Freeman BA: Peroxynitrite oxidation of sulfhydryls: the cytotoxic potential of superoxide and nitric oxide. J Biol Chem 266: 4244–4250, 1991
Radi R, Beckman JS, Bush KM, Freeman BA: Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 288: 481–487, 1991
Beckman JS: The double-edged role of nitric oxide in brain function and superoxidemediated injury. J Dev Physiol 15: 53–59, 1991
Saran M, Michel C, Bors W: Reaction of NO· with O2.-. Implications for the action of endothelium-derived relaxing factor (EDRF). Free Rad Res Com 10: 221–226, 1990
Mahoney LR: Evidence for the formation of hydroxyl radicals in the isomerization of pernitrous acid to nitric acid in aqueous solution. J Am Chem Soc 92: 5262–5263, 1970
Augusto O, Gatti RM, Radi R: Spin-trapping studies of peroxynitrite decomposition and of 3-morpholinosydnonimine N-ethylcarbamide autooxidation: Direct evidence for metal-independent formation of free radical intermediates. Arch Biochem Bioph 310: 118–125, 1994
Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H and Beckman JS: Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol 5: 834–842, 1992
Pryor WA, Squadrito GL: The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am J Physiol 268: L699–L722, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dalloz, F., Maupoil, V., Lecour, S. et al. In vitro studies of interactions of NO· donor drugs with superoxide and hydroxyl radicals. Mol Cell Biochem 177, 193–200 (1997). https://doi.org/10.1023/A:1006821724773
Issue Date:
DOI: https://doi.org/10.1023/A:1006821724773