Abstract
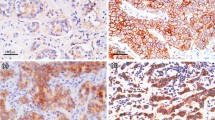
The invasive potential of tumor cells is usually tested either by in vitro invasion assays which evaluate cell spreading ability in basement membrane‐like matrices or by in vivo invasion assays in nude mice. Both methods are laborious and time‐consuming. Tumor invasiveness is accompanied by the changes in expression of various genes. The invasive behavior of cells is therefore represented by certain gene expression patterns. The purpose of this study was to investigate whether expression patterns of several genes are characteristic for the invasiveness of cultured cells. We examined the mRNA levels of estrogen receptor (ER), progesterone receptor (PR), estrogen inducible pS2 and plasminogen activator inhibitor‐1 (PAI‐1) in 23 cell lines derived from benign and malignant breast tissues using a competitive reverse transcription‐polymerase chain reaction (cRT‐PCR) system. We also evaluated the invasiveness of these cell lines by their ability to penetrate into a collagen‐fibroblast matrix. We demonstrate that the gene expression pattern of breast cell lines is clearly associated with its in vitro invasiveness. In general, cells with ER, PR, pS2 but no PAI‐1 expression showed a non‐invasive phenotype, while cells expressing PAI‐1 mRNA but not ER mRNA are invasive. Our study indicates that the invasiveness of breast cancer cell lines is characterized by PAI‐1 gene expression and the lack of ER mRNA. This suggests that PAI‐1 may participate in the invasive process.
Similar content being viewed by others
References
Foekens JA, Schmitt M, Van Putten WLJ, Peters HA, Kramer MD, Jänicke F, Klijn JGM: Plasminogen activator inhibitor-1 and prognosis in primary breast cancer. J Clin Oncol 12: 1648–1658, 1994
Stonelake PS, Baker PG, Gillespie WM, Dunn JA, Spooner D, Morrison JM, Bundred NJ, Oates GD, Lee MJR, Neoptolemos JP, Chan SY, Baker PR: Steroid receptors, pS2 and cathepsin D in early clinically node-negative breast cancer. Eur J Cancer 30: 5–11, 1994
Stal O, Brisfors A, Carstensen J, Ferraud L, Hatschek T, Nordenskjöld B: The South-East Sweden Breast Cancer Group. Relationships of DNA ploidy, S-phase fraction and hormone receptor status to tumor stage in breast cancers detected by population screening. Int J Cancer 51: 28–33, 1992
Alghanem AA, Hussain S: The effect of tumor size and axillary lymph node metastasis on estrogen and progesterone receptors in primary breast cancer. J Surg Oncol 31: 218–221, 1986
Clark GM, McGuire WL: Prognostic factors in primary breast cancer. Breast Cancer Res Treat 3 (Suppl): S69–S72, 1983
McGuire WL: Hormone receptor in breast cancer. In: Bulbrook RD (ed) Cancer Surveys. Vol 5. Oxford University Press, Oxford, 1986, pp 527–536
Garcia M, Derocq D, Freiss G, Rochefort H: Activation of estrogen receptor transfected into a receptor-negative breast cancer cell line decreases the metastatic and invasive potential of the cells. Proc Natl Acad Sci USA 89: 11538–11542, 1992
Masiakowski P, Breathnach R, Bloch J, Gannon F, Krust A, Chambon P: Cloning of cDNA sequences of hormon-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res 10: 7895–7903, 1982
Soubeyran I, Wafflart J, Bonichon F, DeMascarel I, Trojani M, Durand M, Avril A, Coindre JM: Immunohistochemical determination of pS2 in invasive breast carcinomas: a study on 942 cases. Breast Cancer Res Treat 34: 119–128, 1995
Racca S, Conti G, Pietribiasi F, Stramignoni D, Tampellini M, Valetto MR, Ghezzo F, Di Carlo F: Correlation between pS2 protein positivity, steroid receptor status and other prognostic factors in breast cancer. Int J Biol Markers 10: 87–93, 1995
Foekens JA, Rio MC, Seguin P, VanPutten WL, Fauque J, Nap M, Klijn JG, Chambon P: Prediction of relapse and survival in breast cancer patients by pS2 protein status. Cancer Res 50: 3832–3837, 1990
Horiguchi J, Lino Y, Takei H: Expression of pS2 estrogeninducible protein in primary breast cancer. Oncology 53: 12–15, 1996
Liotta LA, Goldfarb RH, Brundage R, Siegal GP, Terranova V, Garbisa S: Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res 41: 4629–4636, 1981
VanMourik JA, Lawrence DA, Loskutoff DJ: Purification of an inhibitor of plasminogen activator (antiactivator) synthesized by endothelial cells. J Biol Chem 259: 14914–14921, 1984
Foucré D, Bouchet C, Hacène K, Pourreau-Schneider N, Gentile A, Martin PM, Desplaces A, Oglobine J: Relationship between cathepsin D, urokinase, and plasminogen activator inhibitors in malignant vs benign breast tumours. Br J Cancer 64: 926–932, 1991
Jänicke F, Schmitt M, Pache L, Ulm K, Harbeck N, Höfler H, Graeff H: Urokinase (uPA) and its inhibitor PAI-1 are strong and independent prognostic factors in node-negative breast cancer. Breast Cancer Res Treat 24: 195–208, 1993
Grøndahl-Hansen J, Christensen IJ, Rosenquist C, Brünner N, Mouridsen HT, Danø K, Blichert-Toft M: High levels of urokinase-type plasminogen activator and its inhibitor PAI-1 in cytosolic extracts of breast carcinomas are associated with poor prognosis. Cancer Res 53: 2513–2521, 1993
Sumiyoshi K, Baba S, Sakaguchi S, Urano T, Takada Y, Takada A: Increase in levels of plasminogen activator and type-1 plasminogen activator inhibitor in human breast cancer: possible roles in tumor progression and metastasis. Thromb Res 63: 59–71, 1991
Sumiyoshi K, Serizawa K, Urano T, Takada Y, Takada A, Baba S: Plasminogen activator system in human breast cancer. Int J Cancer 50: 345–348, 1992
Tong D, Schneeberger C, Leodolter S, Zeillinger R: Quantitative determination of gene expression by competitive reverse transcription-polymerase chain reaction in degraded RNA samples. Anal Biochem 251: 173–177, 1997
Kury FD, Schneeberger C, Sliutz G, Kubista E, Salzer H, Medl M, Leodolter S, Swoboda H, Zeillinger R, Spona J: Determination of HER-2/neu amplification and expression in tumor tissue and cultured cells using a simple, phenol free method for nucleic acid isolation. Oncogene 5: 1403–1408, 1990
Sedlák J, Sedláková O, Hlavcák P, Hunáková L, Bizik J, Grófová M, Chorváth B: Cell surface phenotype and increased penetration of human multidrug-resistant ovarian carcinoma cells into in vitro collagen-fibroblasts matrix. Neoplasma 43: 389–395, 1996
Thompson EW, Paik S, Brünner N, Sommers CL, Zugmaier G, Clarke R, Shima TB, Torri J, Donahue S, Lippman ME, Martin GR, Dickson RB: Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol 150: 534–544, 1992
Sommers CL, Byers SW, Thompson EW, Torri JA, Gelmann EP: Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res Treat 31: 325–335, 1994
Sliutz G, Eder H, Koelbl H, Tempfer C, Auerbach L, Schneeberger C, Kainz C, Zeillinger R: Quantification of uPA receptor expression in human breast cancer cell lines by cRTPCR. Breast Cancer Res Treat 40: 257–263, 1996
Koh KK, Mincemoyer R, Bui MN, Csako G, Pucino F, Guetta V, Waclawiw M, Cannon RO III: Effects of hormonereplacement therapy on fibrinolysis in postmenopausal women. N Engl J Med 336: 683–690, 1997
Meilahn EN, Cauley JA, Tracy RP, Macy EO, Gutai JP, Kuller LH: Association of sex hormones and adiposity with plasma levels of fibrinogen and PAI-1 in postmenopausal women. Am J Epidemiol 143: 159–166, 1996
Dong G, Schulick AH, DeYoung MB, Dichek DA: Identi-fication of a cis-acting sequence in the human plasminogen activator inhibitor type-1 gene that mediates transforming growth factor-b1 responsiveness in endothelium in vivo. JBiol Chem 271: 29969–29977, 1996
Keeton MR, Curriden SA, VanZonneveld AJ, Loskutoff DJ: Identification of regulatory sequences in the type 1 plasminogen activator inhibitor gene responsive to transforming growth factor-beta. J Biol Chem 266: 23048–23052, 1991
Farina AR, Coppa A, Tiberio A, Tacconelli A, Turco A, Colletta G, Gulino A, Mackay AR: Transforming growth factor-beta1 enhances the invasiveness of human MDA-MB-231 breast cancer cells by up-regulating urokinase activity. Int J Cancer 75: 721–730, 1998
Herman ME, Katzenellenbogen BS: Alterations in transforming growth factor-alpha and-beta production and cell responsiveness during the progression of MCF-7 human breast cancer cells to estrogen-autonomous growth. Cancer Res 54: 5867–5874, 1994
Knabbe C, Zugmaier G, Schmahl M, Dietel M, Lippman ME, Dickson RB: Induction of transforming growth factor-beta by the antiestrogens droloxifene, tamoxifen, and toremifene in MCF-7 cells. Am J Clin Oncol 14 (Suppl 2): S15–S20, 1991
Chen H, Tritton TR, Kenny N, Absher M, Chiu JF: Tamoxifen induces TGF-beta 1 activity and apoptosis of human MCF-7 breast cancer cells in vitro. J Cell Biochem 61: 9–17, 1996
Raymond WA, Leong AS: Co-expression of cytokeratin and vimentin intermediate filament proteins in benign and neoplastic breast epithelium. J Pathol 157: 299–306, 1989
Deng G, Curriden SA, Wang S, Rosenberg S, Loskutoff DJ: Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J Cell Biol 134: 1563–1571, 1996
Bajou K, Noel A, Gerard RD, Masson V, Brunner N, Hoist-Hansen C, Skobe M, Fusenig NE, Carmeliet P, Collen D, Foidart JM: Absence of host plasminogen activator inhibitor-1 prevents cancer invasion and vacularization. Nat Med 4: 923–928, 1998
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tong, D., Czerwenka, K., Sedlak, J. et al. Association of in vitro invasiveness and gene expression of estrogen receptor, progesterone receptor, pS2 and plasminogen activator inhibitor‐1 in human breast cancer cell lines. Breast Cancer Res Treat 56, 91–97 (1999). https://doi.org/10.1023/A:1006262501062
Issue Date:
DOI: https://doi.org/10.1023/A:1006262501062