Abstract
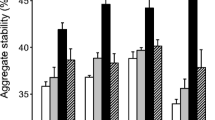
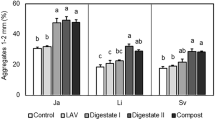
Soil aggregation is a dynamic process in which plants and the soil microbiota play a major role. This experiment was conducted to determine whether the effects of mycorrhizae on the stability of water-stable soil aggregates (WSA) and on selected groups of soil microorganisms are interrelated. Soil containers consisting of four compartments were utilized. Two compartments on each side of a solid barrier were separated by a 43 μm screen that permitted the passage of hyphae, but not of roots. The roots of Sorghum bicolor plants were split over the center barrier, and the roots on one side were inoculated with an arbuscular-mycorrhizal (AM) fungus. This design produced mycorrhizosphere soils (M) by AM roots or hyphosphere (H) soils by AM hyphae in the two compartments on the one side of the barrier, and rhizosphere soils (R) by nonAM roots or root- and hypha-free bulk soil (S) in the two compartments on the other side. At harvest (10 wk), there were significant differences in WSA between soils in the order: M>R>H>S, and WSA stability was significantly correlated with root or hyphal length. Numbers of colony-forming units of the microflora (total bacteria, actinomycetes, anaerobes, P solubilizers, and nonAM fungi) were in general not correlated with root or hyphal length, but in some cases were significantly correlated with WSA. Bacteria isolated from the water-stable soil-aggregate fraction tended to be more numerous than from the unstable fraction. The difference was significant in the M soil for total bacteria and P solubilizing bacteria. NonAM fungi were more numerous in the unstable fraction of the M soil. The data show that the root and fungal components of mycorrhizae enhance WSA stability individually and additively in concert, and suggest that they affect microorganism numbers indirectly by providing a favorable and protective habitat through the creation of habitable pore space in the WSA.
Similar content being viewed by others
References
Andrade G, Azcón R and Bethlenfalvay G J 1995 A rhizobacterium modifies plant and soil responses to the mycorrhizal fungus Glomus mosseae. Appl. Soil Ecol. 2, 195–202.
Andrade G, Mihara K L, Linderman R G and Bethlenfalvay G J 1997 Bacteria from rhizosphere and hyphosphere soils of different arbuscular-mycorrhizal fungi. Plant Soil. 192, 71–79.
Azcón-Aguilar C and Barea J M 1992 Interactions between mycorrhizal fungi and other rhizosphere microorganisms. In Mycorrhizal Functioning. Ed. M F Allen. pp 163–198. Chapman and Hall, NY.
Bagyaraj D J 1984 Biological interactions with VA mycorrhizal fungi. In VA Mycorrhiza. Eds. C Ll Powell and D J Bagyaraj. pp 131–153. CRC Press, Boca Raton, FL.
Bethlenfalvay G J, Andrade G and Azcón-Aguilar C 1997 Plant and soil responses to mycorrhizal fungi and rhizobacteria in nodulated or nitrate-fertilized peas. Biol. Fert. Soils 24, 164–168.
Bresson L M and Moran C J 1995 Structural change induced by wetting and drying in seed beds of a hardsetting soil with contrasting aggregate size distribution. Eur. J. Soil Sci. 46, 205–214.
Burns R G and Davies J A 1986 The microbiology of soil structure. Biol. Agric. Hortic. 3, 95–113.
Christensen H and Jakobsen I 1993 Reduction of bacterial growth by a vesicular-arbuscular mycorrhizal fungus in the rhizosphere of cucumber (Cucumis sativus L.). Biol. Fertil. Soils 15, 253–258.
De Freitas P L, Zobel R W and Snyder V A 1996 A method for studying the effects of soil aggregate size and density. Soil Sci. Soc. Am. J. 60, 288–290.
Drazkiewitz M 1994 Distribution of microorganisms in soil aggregates: Effect of aggregate size. Folia Microbiol. 39, 276–282.
Drazkiewitz M 1996 Generic composition of fungi in soil aggregates. Folia Microbiol. 41, 272–276.
Elliott E T, Anderson R V, Coleman D C and Cole C V 1980 Habitable pore space and microbial trophic interactions. Oikos 35, 327–335.
Elliott E T, Hunt H W, Walter D E and Moore J C 1987 Microcosms, mesocosms and ecosystems: Linking the laboratory to the field. Proc. 4th Intl. Symp. Microb. Ecol. Ljublana, Yugoslavia, 1996.
Emerson WW and Greenland D J 1990 Soil aggregates — formation and stability. In Soil Colloids and their Associations in Aggregates. Eds. M F De Boodt, M B H Hayes and A Herbillon. pp 485–511. Plenum Press, New York.
Foster R C, Rovira A D and Cock T W 1983 Ultrastructure of the Root-Soil Interface. pp 5–11. American Phytopathological Society, St. Paul, MN.
Germida J J and Walley F L 1996 Plant-growth promoting rhizobacteria alter rooting patterns and arbuscular mycorrhizal fungi colonization of field-grown spring wheat. Biol. Fertil. Soils 23, 113–120.
Giovanetti M and Mosse B 1980 An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infections in roots. New Phytol. 84, 489–500.
Graham J H, Linderman R G and Menge J A 1982 Development of external hyphae by different isolates of mycorrhizal Glomus spp. in relation to root colonization and growth of Troyer Citrange. New Phytol. 91, 183–189.
Gryndler M and Vosàtka M 1996 The response of Glomus fistulasum-maize mycorrhiza to treatments with culture fractions from Pseudomonas putida. Mycorrhiza 6, 207–211.
Harris R F, Chester G and Allen O N 1966 Dynamics of soil aggregation. Adv. Agron. 18, 107–169.
Illmer P, Barbato A and Schinner F 1995 Solubilization of hardly-soluble AlPO4 with P-solubilizing microorganisms. Soil Biol. Biochem. 27, 265–270.
Jakobsen I and Rosendahl L 1990 Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New Phytol. 115, 77–83.
Kemper W D and Rosenau R C 1986 Aggregate stability and size distribution. In Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods. Ed. A Klute. pp 425–442. Monograph No. 9, 2nd edn. American Society of Agronomy, Madison, WI.
Kilbertus G 1980 Étude des microhabitats contenus dans les agrégats du sol, leur relation avec la biomasse bactérienne et la taille des procaryotes présents (Study of microhabitats contained in soil aggregates and their relationship with bacterial biomass and the size of the procaryotes present). Rev. Écol. Biol. Sol 17, 543–557.
Kirchner M J, Wollum II, A G and King L D 1993 Soil microbial populations and activities in reduced chemical input agroecosystems. Soil Sci. Soc. Am. J. 57, 1289–1295.
Lynch J M 1981 Promotion and inhibition of soil aggregate stabilization by soil microorganisms. J. Appl. Bacteriol. 126, 371–375.
Martin J P 1950 Use of acid, rose bengal and streptomycin in the plate method for estimating soil fungi. Soil Sci. 69, 215–232.
Meyer J R and Linderman R G 1986 Selective influence on populations of rhizosphere or rhizoplane bacteria and actinomycetes by mycorrhizas formed by Glomus fasciculatum. Soil Biol. Biochem. 18, 191–196.
Miller M and Dick R P 1995 Dynamics of soil C and microbial biomass in whole soil and aggregates in two cropping systems. Appl. Soil Ecol. 2, 253–256.
Miller R M and Jastrow J D 1992 The role of mycorrhizal fungi in soil conservation. In Mycorrhizae in Sustainable Agriculture. Eds. G J Bethlenfalvay and R G Linderman. pp 29–44. American Society of Agronomy, Special Publication No. 54. American Society of Agronomy, Madison, WI.
Molongoski J J and Klug M J 1976 Characterization of anaerobic heterotrophic bacteria isolated from freshwater lake sediments. Appl. Environ. Microbiol. 31, 83–90.
Nehl D B, Allen S J and Brown J F 1996 Deleterious rhizosphere bacteria: an integrating perspective. Appl. Soil Ecol. 5, 1–20.
Nelson L A 1989 A statistical editor's viewpoint of statistical usage in horticultural science publications. HortScience 24, 53–57.
Olsson P A, Baath E, Jakobsen I and Söderström B 1996 Soil bacteria respond to presence of roots but not to mycelium of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 28, 463–470.
Perfect E, Kay B D, van Loon W K P, Sheard R W and Pojasok T 1990 Factors influencing soil structure stability within a growing season. Soil Sci. Soc. Am. J. 54, 173–179.
Schreiner R P, Mihara K L, McDaniel H and Bethlenfalvay G J 1997 Mycorrhizal fungi influence plant and soil functions and interactions. Plant Soil 188, 199–210.
Secilia J and Bagyaraj D J 1987 Bacteria and actinomycetes associated with pot cultures of vesicular-arbuscular mycorrhizas. Can. J. Microbiol. 33, 1067–1073.
Sierra J, Renault P and Valles V 1995 Anaerobiosis in saturated soil aggregates: modeling and experiment. Eur. J. Soil Sci. 46, 519–531.
Sollins P, Homann P and Caldwell B A 1996 Stabilization and destabilization of soil organic matter: mechanisms and controls. Geoderma 74, 65–105.
Sylvester-Bradley R, Akasawa, N, La Torranca S, Magalhaes F M M, Oliveira L A and Pereira RM 1982. Levantamento quantitativo de microorganismos solubilizadores de fosfatos na rizosfera de gramineas e leguminosas forrageiras na Amazonia (Quantitative culturing of phosphate-solubilizing microorganisms in the rhizospheres of gramineous and leguminous forage plants of Amazonia). Acta Amazonica 12, 15–22.
Sylvia D M 1992 Quantification of external hyphae of vesicular-arbuscular mycorrhizal fungi. Methods Microbiol. 24, 53–65.
Thomas R S, Franson R L and Bethlenfalvay G J 1993 Separation of vesicular-arbuscular mycorrhizal fungus and root effects on soil aggregation. Soil Sci. Soc. Am. J. 57, 77–81.
Tisdall J M and Oades J M 1982 Organic matter and water stable aggregates in soils. J. Soil Sci. 33, 141–163.
Wollum II, A G 1982 Cultural methods for soil microorganisms. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties. Eds. A L Page and R H Miller. pp 781–802. Agronomy Monograph No. 9, 2nd Edn. American Society of Agronomy, Madison, WI.
Wright S F and Upadhaya A 1996 Extraction of an abundant and unusual protein from soil and comparison with hyphal protein from arbuscular mycorrhizal fungi. Soil Sci. 161, 575–586.
Zuberer D A 1994 Recovery and enumeration of viable bacteria. In Methods of Soil Analysis, Part 2. Microbiological and Biochemical Properties. Ed. R D Weaver. pp 119–144. Book Series No. 5, Soil Science Society of America, Madison, WI.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Andrade, G., Mihara, K., Linderman, R. et al. Soil aggregation status and rhizobacteria in the mycorrhizosphere. Plant and Soil 202, 89–96 (1998). https://doi.org/10.1023/A:1004301423150
Issue Date:
DOI: https://doi.org/10.1023/A:1004301423150