Abstract
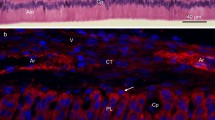
Proteoglycans are complex macromolecules containing one or more glycosaminoglycan chains and exhibiting a variety of biological functions in connective tissues. The aim of the present study was to immunolocalize the distribution of keratan sulphate and chondroitin sulphate epitopes during initial enamel formation in order to study temporo-spatial expression patterns of these macromolecules. Third molars of four-months-old pigs were used for immunolocalization of keratan sulphate and chondroitin sulphate epitopes in the developing enamel layer. Tooth organs were prepared for paraffin sections in order to perform indirect immunohistochemistry. The results demonstrated a mutually exclusive positioning between these two epitopes. Keratan sulphate epitopes were observed in pre-secretory pre-ameloblasts and adjacent stratum intermedium while chondroitin sulphate epitopes were demonstrated in secretory ameloblasts and adjacent stratum intermedium. Our findings suggest that proteoglycans containing glycosaminoglycan chains may play a regulatory role during enamel mineralization.
Similar content being viewed by others
References cited
Caterson B, Christner JE, Baker JR, Couchman JR (1985) Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Fed Proc 44: 386-393.
Chardin H, Septier D, Goldberg M (1990) Visualization of glycosaminoglycans in rat incisor predentin and dentin with cetylpyridinium chloride-glutaraldehyde as fixative. J Histochem Cytochem 38: 885-894.
Cheng H, Caterson B, Neame PJ, Lester GE, Yamauchi M (1996) Differential distribution of lumican and fibromodulin in tooth cementum. Connect Tiss Res 34: 87-96.
Diekwisch T, David S, Bringas P, Santos V, Slavkin HC (1993) Antisense inhibition of AMEL translation demonstrates supramolecular controls for enamel HAP crystal growth during embryonic mouse molar development. Development 117: 471-482.
Diekwisch TGH, Ware J, Fincham AG, Zeichner-David M (1997) Immunohistochemical similarities and differences between amelogenin and tuftelin gene products during tooth development. J Histochem Cytochem 45: 859-866.
Doi Y, Eanes ED, Shimokawa H, Termine JD (1984) Inhibition of seeded growth of enamel apatite crystals by amelogenin and enamelin protein in vitro. J Dent Res 58B: 98-105.
Fincham AG, Lau EC, Simmer J, Zeichner-David M (1992) Amelogenin biochemistry — form and function. In: Slavkin HC, Price P, eds. Chemistry and Biology of Mineralized Tissues. Amsterdam: Excerpta Media, pp. 187-201.
Frank RM, Osman M, Meyer JM, Ruch JV (1979) 3H-glucosamine electron microscope autoradiography after isolated labeling of the enamel organ or the dental papilla followed by reassociated toothgerm culture. J Biol Buccale 7: 225-241.
Galbraith DB, Cutler LS, Kollar EJ (1992) The correlation of temporal regulation of glycosaminoglycan synthesis with morphogenetic events in mouse tooth development. Arch Oral Biol 37: 623-628.
Goldberg M, Septier D (1986) Ultrastructural location of complex carbohydrates in developing rat incisor enamel. Anat Rec 216: 181-190.
Goldberg M, Septier D (1992) Differential staining of glycosaminoglycans in the predentine and dentine of rat incisor using cuprolinic blue at various magnseium chloride concentrations. Histochem J 24: 648-654.
Goldberg M, Lecolle S (1995) Poly-L-lysine-gold complexes used at different pH are probes for differential detection of glycosaminoglycans and phosphoproteins in the predentine and dentine of rat incisor. Histochem J 27: 401-410.
Goldberg M, Septier D, Escaig-Haye F (1987) Glycoconjugates in deninogenesis and dentine. Prog Histochem Cytochem 17: 1-112.
Goldberg M, Chardin H, Septier D, Lecolle S (1993) Proteoglycans—phospholipids interactions: roles in dentine mineralization. C R Seances Soc Biol Fil 187: 210-222.
Heinegard D, Oldberg A (1993) Glycosylated matrix proteins. In: Royce PM, Steinmen B, eds. Connective Tissue and Its Heritable Disorders. New York: Wiley-Liss Inc., pp. 189-209.
Hurmerinta K (1982) Autoradiographic visualization of glycoproteins and glycosaminoglycans in the epithelio-mesenchymal interface of developing mouse tooth germ. Scand J Dent Res 90: 278-285.
Iozzo RV, Murdoch AD (1996) Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J 10: 598-614.
Kakizawa Y, Kasuya K, Kojima N, Nagai S, Takagi H, Fukui K (1976) An histochemical study on acid mucopolysaccharides in the enameloid formation stages of fish (Oplegnathus fasciatus). J Nihon Univ Sch Dent 18: 105-113.
Kogaya Y, Furuhashi K (1985) Ultrastructural distribution of acidic glycosaminoglycans associated with matrix vesicle-mediated calcification in mouse progenitor predentine. Calc Tiss Int 37: 36-41.
Kogaya Y, Furuhashi K (1987) Ultrastructural distribution of sulfated glycosaminoglycans in epithelial—mesenchymal interface of developing rat tooth germs. J Histochem Cytochem 35: 585-593.
Krebsbach PH, Lee SK, Matsuki Y, Kozak CA, Yamada RM, Yamada Y (1996) Full-length sequence, localization, and chromosomal mapping of ameloblastin — a novel tooth-specific gene. J Biol Chem 271: 4431-4435.
Lau EC, Ruch JV (1983) Glycosaminoglycans in embryonic mouse teeth and the dissociated dental constituents. Differentiation 23: 234-242.
Lau E, Arechaga J, Ruch JV (1983a) Glycosaminoglycans in embryonic mouse tooth germs. A histochemical analysis. J Biol Buccale 11: 23-34.
Lau E, Boukari A, Arechaga J, Osman M, Ruch JV (1983b) [35S] autoradiographic study of sulfatedGAGaccumulation and turnover in embryonic mouse tooth germs. J Craniofac Genet Dev Biol 3: 117-131.
Lander AD (1993) Proteoglycans. In: Thomas Kreis, Ronald Vale, eds. Guidebook to the Extracellular Matrix and Adhesion Proteins. Oxford: Oxford University Press. pp. 12-16.
Laurie GW, Leblond CP, Martin GR (1982) Localization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. J Cell Biol 95: 340-344.
Lormee P, Lecolle S, Septier D, Le Denmat D, Goldberg M (1989) Autometallography for histochemical visualization of rat incisor polyanions with cuprolinic blue. J Histochem Cytochem 37: 203-208.
Lyngstadaas SP, Risnes S, Sproat BS, Thrane PS, Prydz HP (1996) A synthetic, chemically-modified ribozyme eliminates amelogenin, the major translation product in developing mouse enamel in vivo. EMBO J 14: 5224-5229.
Mark MP, Baker JR, Kimata K, Ruch J-V (1990a) Regulated changes in chondroitin sulfation during embryogenesis: an immunohistochemical approach. Int J Dev Biol 34: 191-204.
Mark MP, Baker JR, Morrison K, Ruch JV (1990b) Chondroitin sulfates in developing mouse tooth germs. An immunohistochemical study with monoclonal antibodies against chondroitin-4 and chondroitin-6 sulfates. Differentiation 43: 37-50.
Mark MP, Zupan-Bloch A, Ruch JV (1992) Patterned distributions of chondroitin sulfate isoforms, retinoic acid receptor gamma and cellular retinoic acid binding proteins in the embryonic mouse incisor. Proc Finn Dent Soc 88: 439-449.
Ruch JV (1985) Epithelial-mesenchymal interactions. In: Butler WT, ed. The Chemistry and Biology of Mineralized Tissues. Birmingham: EBSCO Media, pp. 54-61.
Ruch JV, Lesot H, Karcher-Djuricic V, Meyer JM, Mark M (1983) Epithelial—mesenchymal interactions in tooth germs: mechanisms of differentiation. J Biol Buccale 11: 173-193.
Sasagawa I (1995) Fine structure of tooth germs during the formation of enameloid matrix in Talapia nilotica, a teleost fish. Arch Oral Biol 40: 801-814.
Sasagawa I (1997) Fine structure of the cap enameloid and of the dental epithelial cells during enameloid mineralization and early maturation stages in the tilapia, a teleost. J Anat 190: 589-600.
Sasaki T (1990) Cell Biology of Tooth Enamel Formation. Basel, Switzerland: Karger, pp. 39-42.
Slavkin HC, Brownell AG, Bringas P, MacDougall M, Bessem C (1983) Basal lamina persistance during epithelial—mesenchymal interactions in murine tooth development in vitro. J Craniofac Gen Dev Biol 3: 387-407.
Thesleff I, Pratt RM (1980) Tunicamycin inhibits mouse tooth morphogenesis and odontoblast differentiation in vitro. J Embryol Exp Morphol 58: 195-208.
Thesleff I, Stenman S, Vaheri A, Timpl R (1979) Changes in the matrix proteins, fibronectin and collagen, during differentiation of mouse tooth germ. Dev Biol 70: 116-126.
Thesleff I, Partanen A-M, Vainio S (1991) Epithelial—mesenchymal interactions in tooth morphogenesis: The roles of extracellular matrix, growth factors, and cell surface receptors. J Craniofac Gen Dev Biol.
Warshawsky H, Moore G (1967) Atechnique for the fixation and decalcification of rat incisors for electron microscopy. J Histochem Cytochem 15: 542-549.
Wight T, Heinegard DK, Hascall VC (1991) Proteoglycans. Structure and function. In: Hay ED, ed. Cell Biology of the Extracellular Matrix. New York: Plenum Press, pp. 45-78.
Zeichner-David M, Vo H, Tan H, Diekwisch T, Berman B, Thiemann F, Alcocer MD, Hsu P, Wang T, Reyna J, Caton J, Slavkin HC, MacDougall M (1997) Timing of the expression of enamel gene products during mouse tooth development. Int J Dev Biol 41: 27-38.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thieberg, R., Yamauchi, M., Satchell, P. et al. Sequential Distribution of Keratan Sulphate and Chondroitin Sulphate Epitopes During Ameloblast Differentiation. Histochem J 31, 573–578 (1999). https://doi.org/10.1023/A:1003871322914
Issue Date:
DOI: https://doi.org/10.1023/A:1003871322914