Abstract
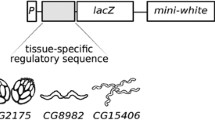
Dosage compensation (equalisation of X-linked gene products) occurs in Drosophila melanogaster by a two-fold transcriptional increase of X-linked gene expression in the male. The cis-acting X-linked DNA sequences required for dosage compensation (called DCREs) remain elusive, despite numerous attempts to identify them. We have developed an insulated reporter system to minimise problems previously encountered with identifying these elements. The system consists of the constitutive autosomal armadillo promoter fused to the lacZ reporter gene (called arm-lacZ) which was flanked by SCS insulator elements to block potential repressive effects of an autosomal chromatin environment. Seven X-linked DNA fragments, totaling 62.7 kb, were each inserted between the SCS element and the armadillo promoter. If an X-linked fragment contains a DCRE, then transgenic males carrying an autosomal insert of the construct should produce twice the ß-galactosidase activity of females. However, in all cases, males and females expressed the same level of lacZ. Thus, it's likely that none of the X-linked fragments contained a DCRE, suggesting these elements may be rarer than previously thought. The insulated reporter system was also used to test the hypothesis that some genes may be dosage compensated due to repression by Sex lethal (Sxl) in females. A fragment from the runt gene containing three Sxl binding sites was inserted into the 3′ untranslated region of arm-lacZ. Transgenic males carrying an autosomal insert of the construct had on average 1.31–1.46 times the level of ß-galactosidase than females, suggesting that some genes could be compensated, at least partially, by Sxl repression in females.
Similar content being viewed by others
References
Amrein, H. & R. Axel, 1997. Genes expressed in neurons of adult male Drosophila. Cell 88: 459–469.
Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith & K. Struhl, 1997. Current Protocols in Molecular Biology. Wiley, New York.
Bashaw, G.J. & B.S. Baker, 1995. The msl-2 dosage compensation gene of Drosophila encodes a putative binding protein whose expression is sex specifically regulated by Sex lethal. Development 121: 3245–3258.
Bashaw, G.J. & B.S. Baker, 1997. The regulation of Drosophila msl-2 gene reveals a function for Sex lethal in translational control. Cell 89: 789–798.
Belote, J.M. & J.C. Lucchesi, 1980. Male specific lethal mutations of Drosophila melanogaster. Genetics 96: 165–186.
Bone, J.R., J. Lavender, R. Richman, M.J. Palmer, B.M. Turner & M.I. Kuroda, 1994. Acetylated histone H4 on the male X chromosome is associated with dosage compensation in Drosophila. Genes Dev. 8: 96–104.
Bossy, B., L.M.C. Hall & P. Spierer, 1984. Genetic activity along 315 kilobases of the Drosophila melanogaster genome. EMBO J. 3: 2537–2541.
Fouts, D., R. Ganguly, A.G. Gutierrez, J.C. Lucchesi & J.E. Manning, 1988. Nucleotide sequence of the Drosophila glucose-6-phosphate dehydrogenase gene and comparison with homologous human gene. Gene 63: 261–275.
Gausz, J., L.M.C. Hall, A. Spierer & P. Spierer, 1984. Molecular genetics of the rosy-Ace region of Drosophila melanogaster. Genetics 112: 65–78.
Gergen, J.P., 1987. Dosage compensation in Drosophila: Evidence that daughterless and Sex-lethal control X chromosome activity at the blastoderm stage of embryogenesis. Genetics 117: 477–485.
Geyer, P.K., 1997. The role of insulator elements in defining domains of gene expression. Curr. Opin. Genet. Dev. 7: 242–248.
Ghosh, S., N. Chatterjee, D. Bunick, J.E. Manning & J.C. Lucchesi, 1989. The LSP-a gene of Drosophila melanogaster exhibits dosage compensation when it is relocated to a different site on the X chromosome. EMBO J. 8: 1191–1196.
Gorman, M., M.I. Kuroda & B.S. Baker, 1993. Regulation of the sex-specific binding of the maleless dosage compensation protein to the male X chromosome in Drosophila. Cell 72: 39–49.
Gu, W., P. Szauter & J. C. Lucchesi, 1998. Targeting of MOF, a putative histone acetyl transferase to the X chromosome of Drosophila melanogaster. Dev. Genet. 22: 56–64.
Gutierrez, A.G., A.C. Christensen, J.E. Manning & J.C. Lucchesi, 1989. Cloning and dosage compensation of the 6-phosphogluconate dehydrogenase gene (Pgd +) of Drosophila melanogaster. Develop. Genet. 10: 155–161.
Hall, L.M., P.J. Mason & P. Spierer, 1984. Transcripts, genes and bands in 315,000 base pairs of Drosophila DNA. J. Mol. Biol. 169: 83–96.
Hazelrigg, T., R. Levis & G.M. Rubin, 1984. Transformation of the white locus DNA in Drosophila: Dosage compensation, zeste interactions and position effects. Cell 36: 469–481.
Hilfiker, A., D. Hilfiker-Kleiner, A. Pannuti & J.C. Lucchesi, 1997. mof, A putative acetyl transferase gene related to the tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila. EMBO J. 16: 2054–2060.
Hilfiker, A., Y. Yang, D.H. Hayes, C.A. Beard, J.E. Manning & J.C. Lucchesi, 1994. Dosage compensation in Drosophila: the X chromosomal binding of MSL-1 and MLE is dependent on Sxl activity. EMBO J. 13: 3542–3550.
Hofmann, A. & G. Korge, 1987. Upstream sequences of dosage compensated and noncompensated alleles of the larval secretion protein gene Sgs-4 in Drosophila melanogaster. Chromosoma 95: 209–215.
Huijser, P., W. Henning & R. Dijkhof, 1987. Poly (dC-dA/dG-dT) repeats in the Drosophila genome: a key function of dosage compensation for a sex linked enzyme in butterflies (Heliconius). Heredity 43: 71–77.
Kelley, R.L., I. Solovyeva, L.M. Lyman, R. Richman, V. Solovyev & M.I. Kuroda, 1995. Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosome and female lethality in Drosophila. Cell 81: 867–877.
Kelley, R.L., J. Wang, L. Bell & M.I. Kuroda, 1997. Sex lethal controls dosage compensation in Drosophila by a nonsplicing mechanism. Nature 387: 195–199.
Kellum, R. & P. Schedl, 1991. A position-effect assay for boundaries of higher order chromosomal domains. Cell 64: 941–950.
Kellum, R. & P. Schedl, 1992. A group of SCS elements function as domain boundaries in an enhancer blocking assay. Mol. Cell Biol. 12: 2424–2431.
Krumm, A., G.E. Roth & G. Korge, 1991. Transformation of salivary gland secretion protein Sgs-4 in Drosophila: stage and tissue specific regulation, dosage compensation and position effect. Proc. Natl. Acad. Sci. USA 82: 5055–5059.
Kuroda, M.I., M.J. Kernan, R. Kreber, B. Ganetsky & B.S. Baker, 1991. The maleless protein associates with the X chromosome to regulate dosage compensation in Drosophila. Cell 66: 935–947.
Levis, R., T. Hazelrigg & G.M. Rubin, 1985. Separable cis-acting control elements for expression of the white gene of Drosophila. EMBO J. 4: 3489–3499.
Levy, L.S. & J.E. Manning, 1981. Messenger RNA sequence complexity and homology in developmental stages of Drosophila. Dev. Biol. 85: 141–149.
Lindsley, D.L. & G.G. Zimm, 1992. The genome of Drosophila melanogaster. Academic Press, New York.
Lowenhaupt, K., A. Rich & M.L. Pardue, 1989. Non-random distribution of long mono-and dinucleotide repeats in Drosophila chromosomes: correlations with dosage compensation, heterochromatin and recombination. Mol. Cell. Biol. 9: 1173–1183.
Lu, L., K.A. Berkey & R.A. Casero, 1996. RGFGIGS is an amino acid sequence required for acetyl coenzyme. A binding and activity of human spermidine/spermine N1 acetyltransferase. J. Biol. Chem. 271: 18920–18924.
Lucchesi, J.C., 1998. Dosage compensation in flies and worms: the ups and downs of X-chromosome regulation. Curr. Opin. Genet. Dev. 8: 179–184.
Lyman, L.M., K. Copps, L. Rastelli, R.L. Kelley & M.I. Kuroda, 1997. Drosophila male-specific-lethal-2 protein: Structure/function analysis and dependence on MSL-1 for chromosome association. Genetics 147: 1743–1753.
Madueno, E., G. Papagiannakis, G. Rimmington, R.D.C. Saunders, C. Savakis, I. Siden-Kiamos, G. Skardvis, L. Spanos, J. Trenear, P. Adam, M. Ashburner, P. Benos, V.N. Bolshakov, D. Coulson, D.M. Glover, S. Herrmen, F.C. Kafatos, C. Louis, T. Majerus & J. Modolell, 1995. A physical map of the X chromosome of Drosophila melanogaster: cosmid contigs and sequence tagged sites. Genetics 139: 1631–1647.
McNabb, S.L. & S.K. Beckendorf, 1986. cis-acting sequences which regulate expression of the Sgs-4 glue protein gene of Drosophila. EMBO J. 5: 2331–2340.
Meller, V.H., K.H. Wu, G. Roman, M.I. Kuroda & R.L. Davis, 1997. roX1 RNA paints the X chromosome of male Drosophila and is regulated by the dosage compensation system. Cell 88: 445–457.
Nüsslein-Volhard, C. & E.F. Wieschaus, 1980. Mutations affecting segment number and polarity in Drosophila embryos. Nature 287: 795–801.
Palmer, M.J., V.A. Mergner, R. Richman, J.E. Manning, M.I. Kuroda & J.C. Lucchesi, 1993. The male specific lethal-one (msl-1) gene of Drosophila melanogaster encodes a novel protein that associates with X chromosomes in males. Genetics 134: 545–557.
Palmer, M.J., R. Richman, L. Richter & M.I. Kuroda, 1994. Sex specific regulation of the male specific lethal-1 dosage compensation gene in Drosophila. Genes Dev. 8: 698–706.
Pardue, M.L., K. Lowenhaupt, A. Rich & A. Nordheim, 1987. (dC–dA)n·(dG–dT)n sequences have evolutionarily conserved chromosomal locations in Drosophila melanogaster with implications for roles in chromosomal structure and function. EMBO J. 6: 1781–1789.
Pirrotta, V., H. Steller & M.P. Bozzetti, 1985. Multiple upstream regulatory elements control the expression of the Drosophila white gene. EMBO J. 4: 3501–3508.
Polito, C., A. Pannuti & J.C. Lucchesi, 1990. Dosage compensation in Drosophila melanogaster: Male and female embryos generated by segregation distortion of the sex chromosomes. Dev. Genet. 11: 249–253.
Qian, S. & V. Pirrotta, 1995. Dosage compensation of the Drosophila white gene requires both the X chromosome environment and multiple intragenic elements. Genetics 139: 733–744.
Riggleman, B., E. Wieschaus & P. Schedl, 1989. Molecular analysis of the armadillo locus: uniformly distributed transcripts and a protein with novel internal repeats are associated with a Drosophila segment polarity gene. Genes Dev. 3: 96–113.
Roehrdanz, R.L., J.M. Kitchens & J.C. Lucchesi, 1976. Lack of dosage compensation for an autosomal gene relocated to the X chromosome in Drosophila melanogaster. Genetics 139: 733–744.
Roseman, R.R., J.M. Swan & P.M. Geyer, 1995. A Drosophila insulator protein facilitates dosage compensation of the X chromosome mini-white gene located at autosomal insertion sites. Development 12: 3473–3582.
Samuels, M.E., D. Bopp, R.A. Colvin, R. Roscigno, M. Garcia-Blanco & P. Schedl, 1994. RNA binding by Sxl proteins in vitro and in vivo. Mol. Cell Biol. 14: 4975–4990.
Scott, M.J. & J.C. Lucchesi, 1991. Structure and expression of the Drosophila melanogaster gene encoding 6-phosphogluconate dehydrogenase. Gene 109: 177–183.
Simon, J.A. & J.T. Lis, 1987. A germline transformation analysis reveals flexibility in the organisation of the heat shock consensus elements. Nucl. Acids Res. 15: 2971–2989.
Spradling, A.G. & G.M. Rubin, 1982. Transposition of cloned P elements into Drosophila germline chromosomes. Science 218: 341–347.
Struhl, K., 1998. Histone acetylation and transcriptional regulatory mechanisms. Genes & Dev. 12: 599–606.
Thummel, C.S., A.M. Boulet & H.D. Lipshitz, 1988. Vectors for Drosophila P-element-mediated transformation and tissue culture transfection. Gene 74: 445–456.
Tobler, J., J.T. Bowman & J.R. Simmons, 1971. Gene modulation in Drosophila: dosage compensation and relocated v + genes. Biochem. Genet. 5: 111–117.
Turner, B.M., A.J. Birley & J. Lavender, 1992. Histone H4 isoforms acetylated at specific residues define individual chromosomes and chromatin domains in Drosophila polytene nuclei. Cell 69: 375–384.
Vazquez, J. & P. Schedl, 1994. Sequences required for enhancer blocking activity of scs are located within two nuclease-hypersensitive sites. EMBO J. 13: 5984–5993.
Vincent, J., C.H. Girdham & P.H. O'Farrell, 1994. A cell-autonomous, ubiquitous marker for the analysis of Drosophila genetic mosaics. Dev. Biol. 164: 328–331.
Zhou, S., Y. Yang, M.J. Scott, A. Pannuti, K.C. Fehr, A. Eisen, E.V. Koonin, D.L. Fouts, R. Wrightsman, J.E. Manning & J.C. Lucchesi, 1995. Male-specific-lethal-2, a dosage compensation gene of Drosophila, undergoes sex specific regulation and encodes a protein with a RING finger and a metallothionein-like cluster. EMBO J. 14: 2884–2895.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fitzsimons, H.L., Henry, R.A. & Scott, M.J. Development of an insulated reporter system to search for cis-acting DNA sequences required for dosage compensation in Drosophila. Genetica 105, 215–226 (1999). https://doi.org/10.1023/A:1003801402153
Issue Date:
DOI: https://doi.org/10.1023/A:1003801402153