Abstract
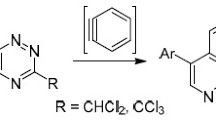
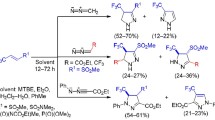
A simple procedure was developed for the synthesis of 1,2,4-triazines and their 4-oxides containing the ClCH2, Cl2CH, or CCl3 group at position 3 by cyclization of 2-aryl-2-hydrazono-1-oximinoethanes with the corresponding chloroacetonitriles. The reaction pathway depends on the number of halogen atoms in the acetonitrile used. The reactions with trichloroacetonitrile, monochloroacetonitrile, and dichloroacetonitrile afford 3-trichloromethyl-1,2,4-triazines, 3-chloromethyl-1,2,4-triazine 4-oxides, and a mixture of the corresponding dichloromethyltriazines and their 4-oxides, respectively. The reactions of 3-trichloromethyl-1,2,4-triazines with indoles and phenols are accompanied by tele-substitution with elimination of halogen from the trichloromethyl group to give 5-indolyl- (or 5-hydroxyphenyl)-3-dichloromethyl-1,2,4-triazines.
Similar content being viewed by others
References
F. Terrier, Nucleophilic Aromatic Displacement:the Influence of the Nitro Group, VCH Publishers, Inc., New York, 1991, 460.
O. N. Chupakhin, V. N. Charushin, and H. C. van der Plas, Nucleophilic Aromatic Substitution of Hydrogen, Academic Press, San Diego, 1994, 246.
M. Makosha, Izv. Akad. Nauk, Ser. Khim., 1996, 531 [Russ. Chem. Bull., 1996, 45, 491 (Engl. Transl.)].
H. C. van der Plas, M. Wozniak, and A. van Veldhuizen, Rec. Trav. Chim., 1978, 97, 130.
E. J. J. Grabowski, E. W. Tristra, R. Tull, and P. I. Pollak, Tetrahedron Lett., 1968, 5931.
R. S. Dainter, T. Jackson, A. H. H. Omar, H. Suschitzky, B. J. Wakefield, N. Hughes, and A. J. Nelson, J. Chem. Soc., Perkin Trans. 1, 1989, 283.
G. Heinisch and T. Huber, Liebigs Ann. Chem., 1992, 19.
T. Giannopoulos, J. R. Ferguso, B. E. Wakefield, and G. Varvounis, Tetrahedron, 2000, 56, 447.
D. N. Kozhevnikov, V. L. Rusinov, and O. N. Chupakhin, Adv. Heterocycl. Chem., 2002, 82, 261.
S. Konno, M. Yokoyama, and H. Yamanaka, Heterocycles, 1982, 19, 1865.
V. L. Rusinov, D. N. Kozhevnikov, E. N. Ulomskii, O. N. Chupakhin, G. G. Aleksandrov, and H. Neunhoeffer, Zh. Org. Khim., 1998, 34, 429 [Russ. J. Org. Chem., 1998, 34, 400 (Engl. Transl.)].
V. L. Rusinov, D. N. Kozhevnikov, I. S. Kovalev, O. N. Chupakhin, and G. G. Aleksandrov, Zh. Org. Khim., 2000, 36, 1081 [Russ. J. Org. Chem., 2000, 36 (Engl. Transl.)].
B. B. Dey, J. Chem. Soc. Londo, 1914, 105
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kozhevnikov, D.N., Kataeva, N.N., Rusinov, V.L. et al. Chloromethyl-, dichloromethyl-, and trichloromethyl-1,2,4-triazines and their 4-oxides: method for the synthesis and tele-substitution reactions with C-nucleophiles. Russian Chemical Bulletin 53, 1295–1300 (2004). https://doi.org/10.1023/B:RUCB.0000042289.07168.8f
Issue Date:
DOI: https://doi.org/10.1023/B:RUCB.0000042289.07168.8f