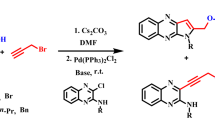
Abstract
Conditions for the regioselective Sonogashira—Hagihara alkynylation of 4-chloro-6-iodo(bromo)quinolines were found and 6-alkynyl-4-chloroquinolines were obtained in 90—100% yields.
Similar content being viewed by others
References
K. Sonogashira, J. Tohda, and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467; K. Sonogashira, Comprehensive Organic Synthesis, Eds. B. M. Trost and I. Fleming, Pergamon Press, Oxford, 1991, 3, 521; K. Sonogashira, Metal-Catalyzed Cross-Coupling Reactions, Eds. F. Diederich and P. J. Stang, Wiley—VCH, Weinheim, 1998, 203; K. Sonogashira, Handbook of Organopalladium Chemistry for Organic Synthesis, Ed. E. Negishi, Wiley—VCH, New York, 2002, 493.
M. P. Lopez-Deber, L. Castedo, and J. R. Granja, Org. Lett., 2001, 3, 2823; D. T. Bong and M. R. Ghadiri, Org. Lett., 2001, 3, 2509.
I. I. Barabanov, L. G. Fedenok, and M. S. Shvartsberg, Izv. Akad. Nauk, Ser. Khim., 1998, 2327 [Russ. Chem. Bull., 1998, 47, 2256 (Engl. Transl.)]; M. S. Shvartsberg, I. I. Barabanov, and L. G. Fedenok, Mendeleev Commun., 1997, 98; L. Anastasia and E.-I. Negishi, Org. Lett., 2001, 3, 3111; U. Radhakrishnan and P. J. Stang, Org. Lett., 2001, 3, 859.
M. Alami, F. Ferri, and G. Linstrumelle, Tetrahedron Lett., 1993, 34, 6403; M. Alami, B. Crousse, and F. Ferri, J. Organomet. Chem., 2001, 624, 114; D. Chemin and G. Linstrumelle, Tetrahedron, 1994, 50, 5335.
R. Menicagli, S. Samaritani, and S. Gori, Tetrahedron Lett., 1999, 40, 8419; M. R. Buchmeiser, T. Schareina, R. Kempe, and K. Wurst, J. Organomet. Chem., 2001, 634, 39.
J. W. Goodby, M. Hird, R. A. Lewis, and K. J. Toyne, Chem. Commun., 1996, 2719; K.-T. Wong, T. S. Hung, Y. Lin, C.-C. Wu, G.-H. Lee, S.-M. Peng, C. H. Chou, and Y. O. Su, Org. Lett., 2002, 4, 513.
H. Nakamura, M. Aizawa, D. Takeuchi, A. Murai, and O. Shimoura, Tetrahedron Lett., 2000, 41, 2185; T. Bach and L. Kruger, Tetrahedron Lett., 1998, 39, 1729; T. Bach and L. Kruger, Synlett, 1998, 1185; T. Bach and L. Kruger, Eur. J. Org. Chem., 1999, 2045.
L.-L. Gundersen, G. Langli, and F. Rise, Tetrahedron Lett., 1995, 36, 1945; G. Langli, L.-L. Gundersen, and F. Rise, Tetrahedron, 1996, 52, 5625; P. Dasa, C. P. Spearsb, A. H. Shahinianb, S. K. Dasguptaa, and N. G. Kundu, Bioorg. Med. Chem. Lett., 1996, 6, 2477.
J. Ellis, E. Gellert, and J. Robson, Aust. J. Chem., 1973, 26, 907; N. J. Leonard and S. N. Boyd, Jr., J. Org. Chem., 1946, 11, 419.
A. V. Tsvetkov, G. V. Latyshev, N. V. Lukashev, and I. P. Beletskaya, Tetrahedron Lett., 2002, 43, 7267.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beletskaya, I.P., Latyshev, G.V., Tsvetkov, A.V. et al. The chemoselective alkynylation of dihaloquinolines by the Sonogashira—Hagihara reaction. Russian Chemical Bulletin 53, 189–193 (2004). https://doi.org/10.1023/B:RUCB.0000024849.57521.49
Issue Date:
DOI: https://doi.org/10.1023/B:RUCB.0000024849.57521.49