Abstract
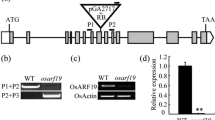
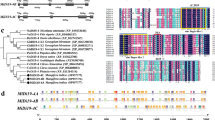
We have isolated a cDNA clone, OsFOR1, from the immature panicles of rice. The OsFOR1 (Oryza sativa floral organ regulator 1) gene encodes a protein that contains a leucine-rich repeat (LRR) domain. This domain comprises 10 tandem repeats of a canonical 24-amino acid LRR sequence. The structure and the number of LRRs for OsFOR1 are similar to those of polygalacturonase-inhibiting proteins (PGIPs) from various other plant species. Moreover, the OsFOR1 recombinant protein, when fused to maltose-binding protein (MBP), shows PGIP activity against the Aspergillus niger polygalacturonase. OsFOR1 is highly expressed in the calli and immature and mature panicles, while detectable at only low levels in seedling roots and mature stems. In situ hybridization experiments showed the transcripts of OsFOR1 are present in young spikelet primordia and in almost all of the young floral organs. Transgenic approaches were used to study in vivo functioning. Antisense expression of OsFOR1 resulted in an increase in the numbers of floral organs, including the stamen, carpel, palea/lemma, stigma, and lodicule. OsFOR1 transcript was not detected in the frizzy panicle mutant, which is defective in its spikelet formation but normal in inflorescence-meristem initiation and maintenance. Therefore, we suggest that OsFOR1 plays a role in the formation and/or maintenance of floral organ primordia.
Similar content being viewed by others
References
An, G., Ebert, P., Mitra, A. and Ha, S. 1988. Binary vectors. In: S.B. Gelvin and R.A. Schilperoort (Eds.) Plant Molecular Biology Manual, Kluwer Academic Publishers, Dordrecht, Netherlands, pp. A3/1–A3/19.
Becraft, P.W. 2002. Receptor kinase signaling in plant development. Annu. Rev. Cell. Dev. Biol. 18: 163–192.
Bonghi, C., Rascio, N., Ramina, A. and Casadoro, G. 1992. Cellulase and polygalacturonase involvement in the abscission of leaf and fruit explants of peach. Plant Mol. Biol. 20: 839–848.
Brownlee, C. 2002. Role of the extracellular matrix in cell-cell signalling: paracrine paradigms. Curr. Opin. Plant Biol. 5: 396–401.
Cervone, F., Hahn, M., de Lorenzo, G., Darvill, A. and Albersheim, P. 1989. Host-pathogen interactions: a plant protein converts fungal pathogenesis factor into an elicitor of plant defence responses. Plant Physiol. 90: 542–548.
Christensen, A., Sharrock, R. and Quail, P. 1992. Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol. Biol. 18: 675–689.
de Lorenzo, G. and Cervone, F. 1997. Polygalacturonase-inhibiting proteins (PGIPs): their role in specificity and defense against pathogenic fungi. In: G. Stacey and N.T. Keen (Eds.) Plant-Microbe Interactions, Vol. 3, Chapman and Hall, New York, pp. 76–93.
de Lorenzo, G., Cervone, F., Bellincampi, D., Caprari, C., Clark, A., Desiderio, A., Devoto, A., Forrest, R., Lockie, F. and Nuss, L. 1994. Polygalacturonase, PGIP and oligogalacturonides in cellcell communication. Biochem. Soc. Trans. 22: 396–399.
de Lorenzo, G., D'Ovidio, R. and Cervone, F. 2001. The role of polygalacturonase-inhibiting proteins (PGIPs) in defense against pathogenic fungi. Annu. Rev. Phytopath. 39: 313–335.
de Veau, E., Gross, K., Huber, D. and Watada, A. 1993. Degradation and solubilization of pectin by β-galactosidases purified from avocado mesocarp. Physiol. Plant. 87: 279–285.
Devoto, A., Leckie, F., Lupotto, E., Cervone, F. and De Lorenzo, G. 1998. The promoter of a gene encoding a polygalacturonaseinhibiting protein of Phaseolus vulgaris L. is activated by wounding but not by elicitors or pathogen infection. Planta 205: 165–174.
Emanuelsson, O., Nielsen, H., Brunak, S. and von Heijne, G. 2000. Predicting subcellular localization of proteins based on their Nterminal amino acid sequence. J. Mol. Biol. 300: 1005–1016.
Favaron, F., D'Ovidio, R., Porceddu, E. and Alghisi, P. 1994. Puri-fication and characterization of a soybean polygalacturonaseinhibiting protein. Planta 195: 80–87.
Favaron, F., Castiglioni, C., D'Ovidio, R. and Alghisi, P. 1997. Polygalacturonase inhibiting proteins from Allium porrum L. and their role in plant tissue against fungal endopolygalacturonases. Physiol. Mol. Plant Path. 30: 403–417.
Fletcher, J.C. 2002. Shoot and floral meristem maintenance in Arabidopsis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 53: 45–66.
Gamboa, A., Paez-Valencia, J., Acevedo, G.F., Vazquez-Moreno, L. and Alvarez-Buylla, R.E. 2001. Floral transcription factor AGAMOUS interacts in vitro with a leucine-rich repeat and an acid phosphatase protein complex. Biochem. Biophys. Res. Commun. 288: 1018–1026.
Hoekema, A., Hirsch, P., Hooykaas, P. and Schilperoort, R. 1983. A binary vector strategy based on separation of vir-and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303: 179–180.
Jang, S., Hong, M.Y., Chung, Y.Y. and An, G. 1999. Ectopic expression of tobacco MADS genes modulates flowering time and plant architecture. Mol. Cells 9: 576–586.
Jeon, J.S., Jang, S., Lee, S., Nam, J., Kim, C., Lee, S.H., Chung, Y.Y., Kim, S.R., Lee, Y.H., Cho, Y.G. and An, G. 2000. leafy hull sterile1 is a homeotic mutation in a rice MADS box gene affecting rice flower development. Plant Cell 12: 871–884.
Jeong, D.H., An, S., Kang, H.G., Moon, S., Han, J.J., Park, S., Lee, H.S., An, K. and An, G. 2003. T-DNA insertional mutagenesis for activation tagging in rice. Plant Physiol. 130: 1636–1644.
Johnston, D., Ramathan, V. and Williamson, B. 1993. A protein from immature raspberry fruits which inhibits endopolygalacturonase from Botrytis cinerea and other micro-organisms. J. Exp. Bot. 44: 971–976.
Kajava, A. 1998. Structural diversity of leucine-rich repeat proteins. J. Mol. Biol. 277: 519–527.
Kang, H.G., Jeon, J.S., Lee, S. and An. G. 1998. Identification of class B and class C floral organ identity genes from rice plants. Plant Mol. Biol. 38: 1012–1029.
Kim, C., Jeong, D. and An, G. 2000. Molecular cloning and characterization of OsLRK1 encoding a putative receptor-like protein kinase from Oryza sativa. Plant Sci. 152: 17–26.
Kobe, B. and Deisenhofer, J. 1995. Protein with leucine-rich repeats. Curr. Biol. 5: 409–416.
Komatsu, M., Maekawa, M., Shimamoto, K. and Kyozuka, J. 2001. The LAX1 and FRIZZY PANICLE 2 genes determine the in-florescence architecture of rice by controlling rachis-branch and spikelet development. Dev. Biol. 231: 364–373.
Kumar, S., Tamura, K., Jakobsen, I.B. and Nei, M. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17: 1244–1245.
Leblanc, C., Falciatore, A., Watanabe, M. and Bowler, C. 1999. Semi-quantitative RT-PCR analysis of photoregulated gene expression in marine diatoms. Plant Mol. Biol. 40: 1031–1044.
Leckie, F., Mattei, B., Capodicasa, C., Hemmings, A., Nuss, L., Aracri, B., de Lorenzo, G. and Cervone, F. 1999. The specificity of polygalacturonase-inhibiting protein (PGIP): a single amino acid substitution in the solvent-exposed beta-strand/beta-turn region of the leucine-rich repeats (LRRs) confers a new recognition capability. EMBO J. 18: 2352–2363.
Lee, S., Jeon, J.S., Jung, K.H. and An, G. 1999. Binary vectors for efficient transformation of rice. J. Plant Biol. 42: 310–316.
Lemaitre, B., Nicolas, E., Michaut, L., Reichhart, J. and Hoffmann, J. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86: 973–983.
Li, R., Rimmer, R., Yu, M., Sharpe, A.G., Seguin-Swartz, G., Lydiate, D. and Hegedus, D.D. 2003. Two Brassica napus polygalacturonase inhibitory protein genes are expressed at different levels in response to biotic and abiotic stresses. Planta 217: 299–308.
Librojo, A. and Khush, G. 1985. Chromosomal location of some mutant genes through the use of primary trisomics in rice. In: Rice Genetics. IRRI, Manila, Philippines, pp. 249–255.
Lim, J., Moon, Y.H., An, G. and Jang, S.K. 2000. Two rice MADS domain proteins interact with OsMADS1. Plant Mol. Biol. 44: 513–527.
Mattei, B., Bernalda, M.S., Federici, L., Roepstorff, P., Cervone, F. and Boffi, A. 2001. Secondary structure and posttranslational modifications of the leucine-rich repeat protein PGIP (polygalacturonase-inhibiting protein) from Phaseolus vulgaris. Biochemistry 40: 569–576.
Meakin, P. and Roberts, J. 1991. Anatomical and biochemical changes associated with the induction of oilseed rape pod dehiscence by Dasineura brassicae. Ann. Bot. 67: 193–197.
Moon, Y.H., Jung, J.Y., Kang, H.G. and An, G. 1999a. Identification of a rice APETALA3 homolog by yeast two-hybrid screening. Plant Mol. Biol. 40: 167–177.
Moon, Y.H., Kang, H.G., Jung, J.Y., Jeon, J.S., Sung, S.K. and An, G. 1999b. Determination of the motif responsible for interaction between rice AP1/AGL9 family proteins using yeast two-hybrid system. Plant Physiol. 120: 1193–1203.
Nagasawa, N., Miyoshi, M., Kitano, H., Satoh, H. and Nagato, Y. 1996. Mutations associated with floral organ number in rice. Planta 198: 627–633.
Pressey, R. 1991. Polygalacturonase in tree pollens. Phytochemistry 30: 1753–1755.
Pressey, R. and Avant, J. 1977. Occurrence and properties of polygalacturonase in Avena and other plants. Plant Physiol. 60: 548–553.
Pressey, R. and Reger, B. 1989. Polygalacturonase in pollen from corn and other grasses. Plant Sci. 59: 57–62
Sander, L., Child, R., Ulvskov, P., Albrechtsen, M. and Borkhardt, B. 2001. Analysis of a dehiscence zone endo-polygalacturonase in oilseed rape (Brassica napus) and Arabidopsis thaliana: evidence for roles in cell separation in dehiscence and abscission zones, and in stylar tissues during pollen tube growth. Plant Mol. Biol. 46: 469–479.
Sentoku, N., Sato, Y., Kurata, N., Ito, Y., Kitano, H. and Matsuoka, M. 1999. Regional expression of the rice KN1-type homeobox gene family during embryo, shoot, and flower development. Plant Cell 11: 1651–1664.
Simpson, C., MacRae, E. and Gardner, R. 1995. Cloning of polygalacturonase-inhibiting protein from kiwifruit. Plant Physiol. 108: 1748.
Sitrit, Y., Downie, B., Bennett, A. and Bradford, K. 1996. A novel exo-polygalacturonase is associated with radicle protrusion in tomato (Lycopersicon esculentum) seeds. (abstract no. 752). Plant Physiol. 111S: 161.
Steinmayr, M., Motte, P., Sommer, H., Saedler, H. and Schwarz-Sommer, Z. 1994. FIL2, an extracellular leucine-rich repeat protein, is specifically expressed in Antirrhinum flowers. Plant J. 5: 459–467.
Stotz, H., Powell, A., Damon, S., Greeve, C., Bennette, A. and Labavitch, J. 1993. Molecular characterisation of a polygalac369 turonase inhibitor from Pyrus communis L. cv. Bartlett. Plant Physiol. 102: 133–138.
Stotz, H., Contos, J., Powell, A., Bennette, A. and Labavitch, J. 1994. Structure and expression of an inhibitor of fungal polygalacturonases from tomato. Plant Mol. Biol. 25: 607–617.
Taylor, J., Tucker, G., Lasslett, Y., Smith, C., Arnold, C., Watson, C., Schuch, W., Grierson, D. and Roberts, J. 1990. Polygalacturonase expression during leaf abscission of normal and transgenic plants. Planta 183: 133–138.
Toubart, P., Desiderio, A., Salvi, G., Cervone, F., Daroda, F., de Lorenzo, G., Bergmann, C, Darvill, A.G. and Albersheim, P. 1992. Cloning and characterisation of the gene encoding the endopolygalact-uronase-inhibiting protein (PGIP) of Phaseolus vulgaris L. Plant J. 2: 367–373.
Weurman, C. 1953. Pectinase inhibitors in pears. Acta Bot. Neerl. 2: 107–121.
Worrall, D., Elias, L., Ashford, D., Smallwood, M., Sidebottom, C., Lillford, P., Telford, J., Holt, C. and Bowles, D. 1998. A carrot leucine-rich-repeat protein that inhibits ice recrystallization. Science 282: 115–117.
Yamaguchi, Y., Mann, D.M. and Ruoslahti, E. 1990. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 346: 281–284.
Yang, K.Y., Jeong, D.H., Jang, S. and An, G. 2001. Molecular cloning and characterization of a rice PP2C, OsPP2C4. J. Plant Biol. 44: 1–6.
Yao, C., Conway, W. and Sams, C. 1995. Purification and characterisation of a polygalacturonase-inhibiting protein from apple fruit. Phytopathology 85: 1373–1377.
Yao, C., Conway, W.S., Ren, R., Smith, D., Ross, G.S. and Sams, C.E. 1999. Gene encoding polygalacturonase inhibitor in apple fruit is developmentally regulated and activated by wounding and fungal infection. Plant Mol. Biol. 39: 1231–1241.
York, W., Darvill, A., McNeil, M., Stevenson, T. and Albersheim, P. 1985. Isolation and characterization of plant cell walls and plant cell wall component. Meth. Enzymol. 118: 3–40.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jang, S., Lee, B., Kim, C. et al. The OsFOR1 gene encodes a polygalacturonase-inhibiting protein (PGIP) that regulates floral organ number in rice. Plant Mol Biol 53, 357–372 (2003). https://doi.org/10.1023/B:PLAN.0000006940.89955.f1
Issue Date:
DOI: https://doi.org/10.1023/B:PLAN.0000006940.89955.f1