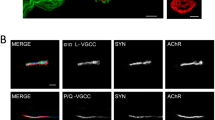
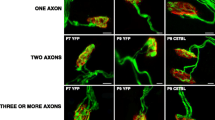
Abstract
We have studied developmental activity-dependent synapse diminution in both an in vitro tissue culture chamber system and at the intact rodent neuromuscular junction (nmj). In both types of preparations, pre- and postsynaptic alterations in synapse structure and function are produced by manipulations of thrombin (Thr) and protein kinase C (PKC) activity. An opposing postsynaptic effect of PKC and protein kinase A (PKA) action on the acetycholine receptor (AChR) can be shown in vitro with PKA stabilizing and PKC destabilizing the nmj synapses. In vivo studies of normal junctional maturation show that changes in axonal inputs and postsynaptic receptor cluster morphology occur, to a substantial degree, independently of one another. Presynaptic actions of PKA are involved in the activity dependent synapse modulation that can be demonstrated in vitro. Late in the elimination process, (>12 days in vivo) the process becomes independent of PKC, implying that diverse, redundant mechanisms are involved in this important developmental process.
Similar content being viewed by others
References
ANGAUT-PETIT, D., MOLGO, J., CONNOLD, A. L. & FAILLE, L. (1987) The Levator auris longus muscle of the mouse:Aconvenient preparation for studies of shortand long term presynaptic effects of drugs or toxins. Neuroscience Lett. 82, 8–88.
BALICE-GORDON, R. J. & LICHTMAN, J. W. (1994) Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature 372, 51–524.
BOURGEOIS, J. & RAKIC, P. (1993) Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J. Neurosci. 13, 280–2820.
BYRNE, J. H. & KANDEL, E. R. (1996) Presynaptic facilitation revisited: State and time dependence. J. Neurosci. 16, 42–435.
CAMBRAY-DEAKIN, M. A., ADU, J. B. & BURGOYNE, R. D. (1990) Neuritogenesis in cerebellar granule cells in vitro, a role for protein kinase C. Dev. Brain Res. 53, 4–46.
CONNOLD, A. L., EVERS, J. V. & VRBOVA, G. (1986) Effect of low calcium and protease inhibitors on synapse elimination during postnatal development in the rat soleus muscle. Brain Res. 393, 9–107.
COUGHLIN, S. R. (2000) Thrombin signalling and proteaseactivated receptors. Nature 407, 25–264.
DAI, Z. & PENG, H. B. (1998) A role of tyrosinepPhosphatase in acetylcholine receptor cluster dispersal and formation. J. Cell. Biol. 141, 161–1624.
FIELDS, R. D. & NELSON, P. G. (1992) Activity-dependent development of the nervous system. Int. Rev. Neurobiol. 34, 13–214.
GIRARD, P. R. & KUO, J. F. (1990) Protein kinase C and its 80-kilodalton substrate protein in neuroblastoma cell neurite outgrowth. J. Neurochem. 54, 30–306.
HUGANIR, R. L. & GREENGARD, P. (1990) Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron 5, 55–567.
JIA, M., LI, M. X., DUNLAP, V. & NELSON, P. G. (1999) The thrombin receptor mediates functional activitydependent neuromuscular synapse reduction via protein kinase C activation in vitro. J. Neurobiol. 38, 36–381.
KANO, M., HASHIMOTO, K., CHEN, C., ABELIOVICH, A., AIBA, A., KURIHARA, H., WATANABE, M., INOUE, Y. & TONEGAWA. S. (1995) Impaired synapse elimination during cerebellar development in PKC gamma mutant mice. Cell 83, 122–1231.
KATZ, B. (1996) Neural transmitter release: From quantal secretion to exocytosis and beyond. The Fenn Lecture. J. Neurocytol. 25, 67–686.
KIM, S., BONDEVA, T. & NELSON, P. G. (2002) Activation of protein kinase C isozymes in primary mouse myotubes by carbachol. Brain Res. Dev. Brain Res. 137, 1–21.
LAI, W. S. & EL-FAKAHANY, E. E. (1988) Regulation of [3H] phorbol-12,13-dibutyrate binding sites in mouse neuroblastoma cells: Simultaneous down-regulation by phorbol esters and desensitization of their inhibition of muscarinic receptor function. J. Pharmacol. Exp. Ther. 244, 4–50.
LANUZA, M. A., GARCIA, N., SANTAFE, M., NELSON, P. G., FENOLL-BRUNET, M. R. & TOMAS, J. (2001) Pertussis toxin-sensitive G-protein and protein kinase C activity are involved in normal synapse elimination in the neonatal rat muscle. J. Neurosci. Res. 63, 33–340.
LANUZA, M. A., GARCIA, N., GONZÀLEZ, C. M., SANTAFÉ, M., NELSON, P. G. & TOMÀS, J. (2003) Role and expression of thrombin receptor PAR-1 in muscle cells and neuromuscular junctions during the synapse elimination period in the neonatal rat. J. Neurosci. Res. 73, 1–21.
LANUZA, M. A., LI, M.-X., JIA, M., KIM, S., DAVENPORT, R., DUNLAP, V. & NELSON, P. G. (2000) Protein kinase C mediated changes in synaptic efficacy at the neuromuscular junction in vitro: The role of postsynaptic acetylcholine receptors. J. Neurosci. Res. 61, 61–625.
LANUZA, M. A., GARCIA, N., SANTAFE, M., GONZALEZ, C. M., ALONSO, I., NELSON, P. G. & TOMAS, J. (2002) Pre-and postsynaptic maturation of the neuromuscular junction during neonatal synapse elimination depends on protein kinase C. J. Neurosci. Res. 67, 60–617.
LI, M.-X., JIA, M., JIANG, H., DUNLAP, V. & NELSON, P. G. (2001) Opposing actions of protein kinase A and C mediate Hebbian synaptic plasticity. Nature Neuroscience 4, 87–872.
LI, M.-X., JIA, M., YANG, L.-X., DUNLAP, V. & NELSON, P. G. (2002) Pre-and postsynaptic mechanisms in Hebbian activity-dependent synapse modification. J. Neurobiol. 52, 24–250.
LIU, Y., FIELDS, R. D., FESTOFF, B. W. & NELSON, P. G. (1994) Proteolytic action of thrombin is required for electrical activity-dependent synapse reduction. Proc. Natl. Acad. Sci. USA 91, 1030–10304.
LOHOF, A. M., DELHAYE-BOUCHAUD, N. & MARIANI, J. (1996) Synapse elimination in the entral nervous system: Functional significance and cellular mechanisms. Rev. Neurosci. 7, 8–101.
MARIANI, J. (1983) Elimination of synapses during the development of the central nervous system. Prog. Brain Res. 58, 38–392.
MATTHIES, H. J., PALFREY, H. C., HIRNING, L. D. & MILLER, R. J. (1987) Down regulation of protein kinase C in neuronal cells: Effects on neurotransmitter release. J. Neurosci. 7, 119–1206.
MILES, K. & HUGANIR, R. L. (1988) Regulation of nicotinic acetylcholine receptors by protein phosphorylation. Mol. Neurobiol. 2, 9–124.
MILES, K., GREENGARD, P., HUGANIR, R. L. (1989) Calcitonin gene-related peptide regulates phosphorylation of the nicotinic acetylcholine receptor in rat myotubes. Neuron 2, 151–1524.
MOHAMED, A. S., RIVAS-PLATA, K. A., KRAAS, J. R., SALEH, S. M. & SWOPE, S. L. (2001) Src-class kinases act within the agrin/MuSK pathway to regulate acetylcholine receptor phosphorylation, cytoskeletal anchoring, and clustering. J. Neurosci. 21, 380–3818.
NIMNUAL, A. S., CHANG, W., CHANG, N. S., ROSS, A. F., GELMAN, M. S. & PRIVES, J. M. (1998) Identification of phosphorylation sites on AChR delta-subunit associated with dispersal of AChR clusters on the surface of muscle cells. Biochemistry 37, 1482–14832.
PERKINS, G. A., WANG, L., HUANG, L. J., HUMPHRIES, K., YAO, V. J., MARTONE, M., DEERINCK, T. J., BARRACLOUGH, D. M., VIOLIN, J. D., SMITH, D., NEWTON, A., SCOTT, J. D., TAYLOR, S. S. & ELLISMAN, M. H. (2001) PKA, PKC, and AKAP localization in and around the neuromuscular junction. BMC Neurosci. 2, 17.
REDFERN, P. A. (1970) Neuromuscular transmission in new-born rats. J. Physiol. (Lond.) 209, 70–709.
REIST, N. E., WERLE, M. J. & MCMAHAN, U. J. (1992) Agrin released by motor neurons induces the aggregation of acetylcholine receptors at neuromuscular junctions. Neuron 8, 86–868.
ROCHE, K. W., TINGLEY, W. G. & HUGANIR, R. L. (1994) Glutamate receptor phosphorylation and synaptic plasticity. Curr. Opin. Neurobiol. 4, 38–388.
SLATER, C. R. (1982) Postnatal maturation of nerve-muscle juncctions in hindlimb muscles of the mouse. Dev. Biol. 94, 1–22.
SWOPE, S. L., MOSS, S. J., BLACKSTONE, C. D. & HUGANIR, R. L. (1992) Phosphorylation of ligandgated ion channels: A possible mode of synaptic plasticity. FASEB J. 6, 251–2523.
THOMPSON, W. J. (1985) Activity and synapse elimination at the neuromuscular junction. Cell Mol. Neurobiol. 5, 16–182.
VRBOVA, G., LOWRIE, M. B. & EVERS, J. (1988) Reorganization of synaptic inputs to developing skeletal muscle fibres. Ciba Found Symp. 138, 13–151.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nelson, P.G., Lanuza, M.A., Jia, M. et al. Phosphorylation reactions in activity-dependent synapse modification at the neuromuscular junction during development. J Neurocytol 32, 803–816 (2003). https://doi.org/10.1023/B:NEUR.0000020625.70284.a6
Issue Date:
DOI: https://doi.org/10.1023/B:NEUR.0000020625.70284.a6