Abstract
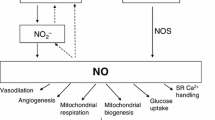
The possible role of nitric oxide on the exercise-induced changes in bleomycin-detectable iron (BDI) in the liver, spleen, bone marrow cells and heart was investigated. Female Sprague—Dawley rats were randomly assigned to four groups: S1 (Sedentary), S2 (Sedentary + L-NAME [N-nitro-L-arginine methyl ester]), E1 (Exercise) and E2 (Exercise + L-NAME). Animals in the E1 and E2 swam for 2 h/day for 3 months. L-NAME in the drinking water (1 mg/ml) was administrated to rats in the S2 and E2 groups for the same period. At the end of the 3rd month, nitrite and nitrate (NOx), BDI and non-heme iron (NHI) contents in the liver, spleen, bone marrow cells and heart were measured. The ratio of BDI/NHI was calculated. The exercise induced a significant increase in NOx and BDI contents and/or BDI/NHI ratio in the spleen, bone morrow cells and heart. Treatment with L-NAME, an inhibitor of NOS, led to a significant decrease in NOx and an increase in BDI levels and BDI/NHI ratios in these tissues. The correlative analysis showed that there is significantly positive correlation between NOx levels and BDI contents and/or BDI/NHI ratios in the spleen, bone marrow cells and heart. These results suggest that the increased nitric oxide might be one of the reasons leading to the increased BDI levels in these tissues in the exercised rats. In contrast to the above tissues, in the liver, exercise led to a significant decrease rather than increase in BDI levels and BDI/NHI ratios with a significant increase in NOx contents. Treatment with L-NAME led to a significant increase in BDI levels and BDI/NHI ratios and a decrease in NOx contents in the tissue. These findings plus the results reported by others imply that nitric oxide might have an inhibitory effect on BDI in the liver.
Similar content being viewed by others
References
Burkitt MJ, Milne L, Raafat A: A simple, highly sensitive and improved method for the measurement of bleomycin-detectable iron: The ‘catalytic iron index’ and its value in the assessment of iron status in haemochromatosis. Clin Sci (Lond) 100: 239-247, 2001
Cabantchik ZI, Glickstein H, Milgram P, Breuer W: A fluorescence assay for assessing chelation of intracellular iron in a membrane model system and in mammalian cells. Anal Biochem 233: 221-227, 1996
Cairo G, Pietrangelo A: Iron regulatory proteins in pathobiology. Biochem J 352: 241-250, 2000
Childs A, Jacobs C, Kaminski T, Halliwell B, Leeuwenburgh C: Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Rad Biol Med 31: 745-753, 2001
Drapier JC, Hirling H, Wietzerbin J, Kaldy P, Kuhn LC: Biosynthesis of nitric oxide activates iron regulatory factors in macrophages. EMBO J 12: 3643-3649, 1993
Epsztejn S, Kakhlon O, Glickstein H, Breuer W, Cabantchik I: Fluorescence analysis of the labile iron pool of mammalian cells. Anal Biochem 248: 31-40, 1997
Esposito BP, Epsztejn S, Breuer W, Cabantchik, ZI: A review of fluorescence methods for assessing labile iron in cells and biological fluids. Anal Biochem 304: 1-18, 2002
Gagne CM, Walberg-Rankin JL, Ritchey SJ: Effects of exercise on iron status in mature female rats. Nutr Res 14: 211-219, 1994
Gutteridge JM, Rowley DA, Halliwell B: Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of ‘free’ iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem J 199: 263-265, 1981
Halliwell B, Gutteridge JMC: Oxygen free redicals and iron in relation to biology and medicine: Some problems and concepts. Arch Biochem Biophys 246: 501-514, 1986
Hentze MW, Kuhn LC: Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc Natl Acad Sci USA 93: 8175-8182, 1996
Ho KP, Xiao DS, Ke Y, Qian ZM: The decreased activity of cytosolic aconitase in the liver, spleen and bone marrow in the exercised rats. Biochem Biophys Res Comm 282: 264-267, 2001
Jenkins RR, Krause K, Schofield LS: Influence of exercise on clearance of oxidant stress products and loosely bound iron. Med Sci Sports Exerc 25: 213-217, 1993
Kaldor I: Studies on intermediary iron metabolism. XII. The measurement of the iron derived from water soluble and water insoluble nonhaem compounds (ferritin and haemosiderin iron) in liver and spleen. Aust J Exp Biol 36: 173-182, 1958
Klausner RD, Rouault TA, Harford JB: Regulating the fate of mRNA: The control of cellular iron metabolism. Cell 72: 19-28, 1993
Konijn AM, Glickstein H, Vaisman B, Meyron-Holtz EG, Slotki IN, Cabantchik ZI: The cellular labile iron pool and intracellular ferritin in K562 cells. Blood 94: 2128-2134, 1999
Mills PC, Smith NC, Casas I, Patricia H, Harris RC, Marlin DJ: Effects of exercise intensity and environmental stress on indices of oxidative stress and iron homeostasis during exercise in the horse. Eur J Appl Physiol 74: 60-66, 1996
Oliveira L, Bouton C, Drapier JC: Thioredoxin activation of iron regulatory proteins. J Biol Chem 274: 516-521, 1999
Petrat F, de Groot H, Rauen U: Subcellular distribution of chelatable iron: A laser scanning microscopic study in isolated hepatocytes and liver endothelial cells. Biochem J 356: 61-69, 2001
Prasad MK, Pratt CA: The effects of exercise and two levels of dietary iron on iron status. Nutr Res 10: 1273-1283, 1990
Qian ZM, Xiao DS, Tang PL, Yao FYD, Liao QK: The increased expression of transferrin receptor on the membrane of erythroblast in strenuous exercised rats. J Appl Physiol 87: 523-529, 1999
Qian ZM, Xiao DS, Tang PL: Changes of transferrin-free iron uptake by bone marrow erythroblasts in the strenuously exercised rats. J Nutr Biochem 11: 367-373, 2000
Qian ZM, Xiao DS, Ke Y, Liao QK: The increased nitric oxide is one of the causes for the changes in iron metabolism in the exercised rats. Am J Physiol 280: R739-R743, 2001
Qian ZM, Xiao DS, Ke Y, Tang PL: Effect of different duration of strenuous exercise on transferrin-bound iron uptake by bone marrow erythroblast in rats. J Nutr Biochem 13: 47-54, 2002
Qian ZM: Nitric oxide and changes of iron metabolism in exercise. Biol Rev 77: 529-536, 2002
Reif DW, Simmons RD: Nitric oxide mediates iron release from ferritin. Arch Biochem Biophys 283: 537-541, 1990
Rodriguez JM, Huberman A: Improved method for the determination of nonheme iron in animal tissues. I. Methodology. Rev Invest Clin 26: 169-173, 1974
Ruckman KS, Sherman AR: Effects of exercise on iron and copper metabolism in rats. J Nutr 111: 1593-1601, 1981
Sergent O, Anger JP, Lescoat G, Pasdeloup N, Cillard P, Cillard J: EPR determination of low molecular weight iron content applied to whole rat hepatocytes. Cell Mol Biol 43: 793-800, 1997
Sjödin B, Westling YH, Apple FS: Biochemical mechanisms for oxygen free redical formation during exercise. Sports Med 10: 236-254, 1990
Sohal RS, Wennberg-Kirch E, Jaiswal K, Kwong LK, Forster MJ: Effect of age and caloric restriction on bleomycin-chelatable and nonheme iron in different tissues of C57BL/6 mice. Free Radic Biol Med 27: 287-293, 1999
Staubli A, Boelsterli UA: The labile iron pool in hepatocytes: Prooxidant-induced increase in free iron precedes oxidative cell injury. Am J Physiol 274: G1031-1037, 1998
Strause L, Hegenaner J, Saltman P: Effects of exercise on iron metabolism in rats. Nutr Res 3: 79-89, 1983
Wardrop SL, Watts RN, Richardson DR: Nitrogen monoxide activates iron regulatory protein 1 RNA-binding activity by two possible mechanisms: Effect on the [4Fe-4S] cluster and iron mobilization from cells. Biochemistry 39: 2748-2758, 2000
Weiss G, Goossen B, Doppler W, Fuchs D, Pantopoulos K, Werner-Felmayer G, Wachter H, Hentze MW: Translational regulation via iron responsive elements by the nitric oxide/NO-synthase pathway. EMBO J 12: 3651-3657, 1993
Werner ER, Werner-Felmayer G, Wachter H, Mayer B: Biosynthesis of nitric oxide: Dependence on pteridine metabolism. Rev Physiol Biochem Pharmacol 127: 97-135, 1996
Xiao DS, Qian ZM: Negative correlation of plasma nitric oxide (NO) and iron levels in the exercised rats. Mol Cell Biochem 208: 163-166, 2000
Xie M, Hermann A, Richter K, Engel E, Kerschbaum HH: Nitric oxide up-regulates ferritin mRNA level in snail neurons. Eur J Neurosci 13: 1479-1486, 2001
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Xiao, D.S., Ho, K.P. & Qian, Z.M. Nitric oxide inhibition decreases bleomycin-detectable iron in spleen, bone marrow cells and heart but not in liver in exercise rats. Mol Cell Biochem 260, 31–37 (2004). https://doi.org/10.1023/B:MCBI.0000026048.93795.03
Issue Date:
DOI: https://doi.org/10.1023/B:MCBI.0000026048.93795.03