Abstract
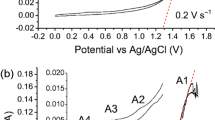
The performance of a batch undivided electrochemical reactor with a rotating cylinder electrode of expanded metal sheets for the removal of tin from synthetic sulfate solution is studied. The effect of the cathode potential, initial tin concentration, number of sheets forming the cathode and cathodic side reactions on the figures of merit of the reactor is analysed. For a cathode potential of −0.65 V vs SCE at 500 rpm, the tin concentration decreased from 393 to 94 mg l−1 after 30 min of electrolysis with a specific energy consumption of 3.93 kWh kg−1 and a normalized space velocity of 1.27 h−1. The change in concentration was higher when the potential was more negative because of the turbulence-promoting action of the hydrogen evolution. The results suggest that the applied potential must represent a compromise between the increase in space time yield or normalized space velocity and the increase in the specific energy consumption.
Similar content being viewed by others
References
K. Inoue, R.S. Mirvaliev, K. Yoshizuka, K. Ohto and S. Babasaki, Solvent Extr. Res. Dev., Jpn. 8 (2001) 21.
D.C. Szlag and N.J. Wolf, Clean Prod. Process. 1 (1999) 117.
A.J. Chaudhary, S.O.V. Dando and S.M. Grimes, J. Chem. Technol. Biotechnol. 76 (2001) 47.
F.C. Walsh, in D. Genders and N. Weinberg (Eds), 'Electrochemistry for a Cleaner Environment' (The Electrosynthesis Company, New York, 1992), pp. 101–159.
G. Kreysa and R. Brandner, in 'Modern Concepts in Electrochemical Reactor Design', Extended Abstracts of the 31st ISE Meeting, Venice, Italy, 2 (1980) H8.
A.H. Nahlé, G.W. Reade and F.C. Walsh, J. Appl. Electrochem. 25 (1995) 450.
E. Merck (Ed.), 'Métodos complexométricos de valoración con titriplex' (Complexometric methods of titration with titriplex), 3rd edn, (A.G. Merck, Darmstadt), p. 34 (in Spanish).
C.N. Reilley and A.J. Barnard Jr, in L. Meites (Ed.), 'Handbook of Analytical Chemistry' (McGraw-Hill, New York, 1963), pp. 3–188.
E.W. Abel, in J.C. Bailar Jr, H.J. Emeléus, R. Nyholm and A.F. Trotman-Dickenson (Eds), 'Comprehensive Inorganic Chemistry' Vol. 2 (Pergamon, Oxford, 1973), pp. 43–104.
J.M. Grau and J.M. Bisang, J. Chem. Technol. Biotechnol. 78 (2003) 1032.
G. Kreysa, DECHEMA Monographs 94 (1983) 123 (in German).
G. Kreysa, Chem.-Ing.-Tech. 55 (1983) 23 (in German).
D.R. Gabe and F.C. Walsh, J. Appl. Electrochem. 14 (1984) 565.
D. Robinson and F.C. Walsh, Hydrometallurgy 26 (1991) 115.
M. Eisenberg, C.W. Tobias and C.R. Wilke, J. Electrochem. Soc. 101 (1954) 306.
F.S. Holland, Chem. and Ind. (London). July (1978) 453.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bazan, J., Bisang, J. Electrochemical Removal of Tin from Dilute Aqueous Sulfate Solutions using a Rotating Cylinder Electrode of Expanded Metal. Journal of Applied Electrochemistry 34, 501–506 (2004). https://doi.org/10.1023/B:JACH.0000021894.86112.63
Issue Date:
DOI: https://doi.org/10.1023/B:JACH.0000021894.86112.63