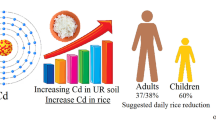
Abstract
We believe greater consideration should be given the agronomic and nutritional/bioavailability factors that influence risk from Cd-contaminated soils. We have argued that the ability of rice to accumulate soil Cd in grain while excluding Fe, Zn and Ca (even though the soil contains 100-times more Zn than Cd) was important in adverse effects of soil Cd is farm families in Asia. Further, polished rice grain is deficient in Fe, Zn and Ca for humans, which promotes Cd absorption into duodenal cells. New kinetic studies clarified that dietary Cd is absorbed into duodenum enterocytes; 109Cd from a single meal remained in the duodenum for up to 16 days; part of the turnover pool 109Cd moved to the liver and kidneys by the end of the 64-day `chase' period. Thus malnutrition induced by subsistence rice diets caused a higher absorption of dietary Cd and much higher potential risk from soil Cd than other crops. Because rice-induced Fe-Zn-Ca-malnutrition is so important in soil Cd risk, it seems evident that providing nutritional supplements to populations of exposed subsistence rice farmers could protect them against soil Cd during a period of soil remediation. In the long term, high Cd rice soils need to be remediated. Remediation by removal and replacement of contaminated soil is very expensive (on the order of $3 million/ha); while phytoextraction using the high Cd accumulating ecotypes of the Zn-Cd hyperaccumulator, Thlaspi caerulescens, should provide low cost soil Cd remediation.
Similar content being viewed by others
References
Ae N, Arao T. 2002 Utilization of rice plants for phytoremediation in heavy metal polluted soils. Farming Japan 36(6), 16-21.
Baker DE, Bowers ME. 1988 Health effects of cadmium predicted from growth and composition of lettuce grown in gardens con-taminated by emissions from zinc smelters. Trace Subst Environ Health 22, 281–295.
Bray BJ, Dowdy RH, Goodrich RD, Pamp DE. 1985 Trace metal accumulations in tissues of goats fed silage produced on sewage sludge-amended soil. J Environ Qual 14, 114–118.
Bouis HE. 2002 Plant breeding: A new tool for fighting micronutri-ent malnutrition. J Nut r132, 491S–494S.
Cai S, Yue L, Hu Z, Zhong X, Ye Z, Xu H, Liu Y, Ji R, Zhang W, Zhang F. 1990 Cadmium exposure and health effects among residents in an irrigation area with ore dressing wastewater. Sci Total Environ 90, 67–73.
Chaney RL, Li Y-M, Angle JS, Baker AJM, Reeves RD, Brown SL, Homer FA, Malik M, Chin M. 2000 Improving metal hyper-accumulator wild plants to develop commercial phytoextraction systems: Approaches and progress. In: Terry N, Banuelos GS, eds. Phytoremediation of Contaminated Soil and Water.Boca Raton, FL: CRC Press: 131–160.
Chaney RL, Ryan JA, Li Y-M, Brown SL. 1999 Soil cadmium as a threat to human health. In: McLaughlin MJ, and Singh BR. eds. Cadmium in Soils and Plants. Dordrecht: Kluwer Academic Publ: 219–256.
Chaney RL, Stoewsand GS, Furr AK, Bache, CA, Lisk DJ. 1978 Elemental content of tissues of guinea pigs fed Swiss chard grown on municipal sewage sludge-amended soil. J Agr Food Chem 26, 994–997.
Ewers U, Freier I, Turfeld M, Brockhaus A, Hofstetter I, König W, Leisner-Saaber J, Delschen T. 1993 Heavy metals in garden soil and vegetables from private gardens located in lead/zinc smelter area and exposure of gardeners to lead and cadmium (in German). Gesundheitswesen 55, 318–325.
Ezaki T, Tsukahara T, Moriguchi J, Furuki K, Fukui Y, Ukai H, Okamoto S, Sakurai H, Honda S, Ikeda M. 2003 No clear-cut evidence for cadmium-induced renal tubular dysfunction among over 10,000 women in the Japanese general population: a na-tionwide large-scale survey. Int Arch Occup Environ Health 76, 186–196.
Flanagan PR, McLellan JS, Haist J, Cherian MG, Chamberlain MJ, Valberg LS. 1978 Increased dietary cadmium absorption in mice and human subjects with iron deficiency. Gastroenterol 74, 841–846.
Fox MRS. 1988 Nutritional factors that may influence bioavailabil-ity of cadmium. J Environ Qual 17, 175–180.
Ikeda M, Ezaki T, Tsukahara T, Moriguchi J, Furuki K, Fukui Y, Ukai H, Okamoto S, Sakura H. 2003 Threshold levels of urin-ary cadmium in relation to increases in urinary â 2-microglobulin among general Japanese populations. Toxicol Lett 137, 135–141.
Kobayashi E, Okubo Y, Suwazono Y, Kido T, Nogawa, K. 2002 Dose-response relationship between total cadmium intake calcu-lated from the cadmium concentration in rice collected from each household of farmers and renal dysfunction in inhabitants of theJinzu River basin, Japan J Appl Toxicol 22, 431–436.
Kobayashi J. 1978 Pollution by cadmium and the itai-itai dis-ease in Japan. pp. 199–260. In Toxicity of Heavy Metals in theEnvironment. Oehme FW Ed. Marcel Dekker, Inc., New York.
Reeves PG, Chaney RL. 2001 Mineral nutrient status of female rats affects the absorption and organ distribution of cadmium from sunflower kernels (Helianthus annuus L.) Environ Res 85, 215–225.
Reeves PG, Chaney RL. 2002 Nutritional status affects the absorp-tion and whole-body and organ retention ot cadmium in rats fed rice-based diets. Environ Sci Technol 36, 2684–2692.
Reeves PG, Chaney RL. 2004 Marginal nutritional status ot zinc, iron, and calcium increases cadmium retention in the duodenum and other organs of rats fed a rice-based diet. Environ Res In press
Sarasua SM, McGeehin MA, Stallings FL, Terracciano GJ, Amler RW, Logue RN, Fox JM. 1995 Final Report. Technical As-sistance to the Pennsylvania Department ot Health. Biologic indicators ot exposure to cadmium and lead. Palmerton, PA. Part II. (May 1995). 57 pp. Agency for Toxic Substances and Disease Registry, US-DHHS, Atlanta, GA, US.
Sharma RP, Kjellström T, McKenzie JM. 1983 Cadmium in blood and urine among smokers and non-smokers with high cadmium intake via food. Toxicol 29, 163–171.
Simmons RW, Pongsakul P, Chaney RL, Saiyasitpanich D, Klin-phoklap S, Nobuntou W. 2003 The relative exclusion ot zinc and iron from rice grain in relation to rice grain cadmium as com-pared to soybean: Implications for human health. Plant Soil 257, 163–170.
Strehlow CD, Barltrop D. 1988 The Shipham Report: An investiga-tion into cadmium concentrations and its implications for human health: 6. Health studies. Sci Total Environ 75, 101–133.
Vahter M, Berglund M, Nermell B, Akesson A. 1996 Bioavailability of cadmium from shelltish and mixed diet in women. Toxicol Appl Pharmacol 136, 332–341.
Welch RM and Graham D. 2002 Breeding crops for enhanced micronutrient content. Plant Soil 245, 205–214.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chaney, R.L., Reeves, P.G., Ryan, J.A. et al. An improved understanding of soil Cd risk to humans and low cost methods to phytoextract Cd from contaminated soils to prevent soil Cd risks. Biometals 17, 549–553 (2004). https://doi.org/10.1023/B:BIOM.0000045737.85738.cf
Issue Date:
DOI: https://doi.org/10.1023/B:BIOM.0000045737.85738.cf