Abstract
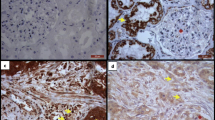
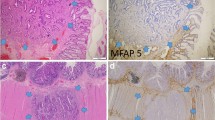
Several matrix metalloproteinases (MMPs) have been implicated in intestinal inflammation, mucosal wound healing, and cancer progression. The purpose of this study was to examine the cellular location and putative function of MMP-19, MMP-26 (matrilysin-2), and MMP-28 (epilysin), in normal, inflammatory, and malignant conditions of the intestine. Peroperative tissue specimens from patients with ulcerative colitis (UC) (n = 16) and archival tissue samples of ischemic colitis (n = 9), Crohn's disease (n = 7), UC (n = 8), colon cancer (n = 20), and healthy intestine (n = 5) were examined using immunohistochemical analyses with polyclonal antibodies. Unlike many classical MMPs, MMP-19, MMP-26, and MMP-28 were all expressed in normal intestine. In inflammatory bowel disease (IBD), MMP-19 was expressed in nonmigrating enterocytes and shedding epithelium. MMP-26 was detected in migrating enterocytes, unlike MMP–28. In colon carcinomas, MMP-19 and MMP-28 expression was downregulated in tumor epithelium. Staining for MMP-26 revealed a meshwork-like pattern between cancer islets, which was absent from most dedifferentiated areas. Our results suggest that MMP-19 is involved in epithelial proliferation and MMP-26 in enterocyte migration, while MMP-28 expression is not associated with inflammatory and destructive changes seen in IBD. In contrast to many previously characterized MMPs, MMP-19 and MMP-28 are downregulated during malignant transformation of the colon and may play a prominent role in tissue homeostasis.
Similar content being viewed by others
References
Nagase H, Woessner J: Matrix metalloproteinases. J Biol Chem 274:21491-21494, 1999
Uria J, López-Otin C: Matrilysin-2, a new matrix metalloproteinase expressed in human tumors and showing the minimal domain organization required for secretion, latency and activity. Cancer Res 60:4745-4751, 2000
Lohi JL, Wilson CL, Roby JD, Parks WC: Epilysin: A novel human matrix metalloproteinase (MMP-28) expressed in testis and keratinocytes and in response to injury. J Biol Chem 276:10134-10144, 2001
Ahokas K, Lohi J, Lohi H, Elomaa O, Karjalainen-Lindsber ML, Kere J, Saarialho-Kere U: Matrix metalloproteinase-21, the human orthologue for XMMP, is expressed during fetal development and in cancer. Gene 301:31-41, 2002
Edwards DR, Beaudry PP, Laing TD, Kowal V, Leco KJ, Leco PA, Lim MS: The roles of tissue inhibitors of metalloproteinases in tissue remodelling and cell growth. Int J Obes Relat Metab Disord 3:9-15, 1996
Gomez D, Alonso DF, Yoshiji H, Thorgeirsson UP: Tissue inhibitors of metalloproteinases: structure, regulation and biological function. Eur J Cell Biol 74:111-122, 1997
Schonbeck U, Mach F, Libby PJ: Generation of biologically active IL-1beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol 161:3340-3346, 1998
Haro H, Crawford HC, Fingleton B, Shinomiya K, Spengler DM, Matrisian LM: Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest 105:143-150, 2000
Bresnihan B: Pathogenesis of joint damage in rheumatoid arthritis. J Rheumatol 26:717-719, 1999
Ishiguro N, Ito T, Ito H, Iwata H, Jugessur H, Ionescu M, Poole AR: Relationship of matrix metalloproteinases and their inhibitors to cartilage proteoglycan and collagen turnover: analyses of synovial fluid from patients with osteoarthritis. Arth Rheum 42:129-136, 1999
Westermarck J, Kähäri VM: Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J 8:781-792, 1999
Schonbeck U, Mach F, Sukhova GK, Murphy C, Bonnefoy JY, Fabunmi RP, Libby P: Regulation of matrix metalloproteinase expression in human vascular smooth muscle cells by T lymphocytes: a role for CD40 signaling in plaque rupture? Circ Res 81:448-454, 1997
Saarialho-Kere UK: Patterns of matrix metalloproteinase and TIMP expression in chronic ulcers. Arch Dermatol Res 90290:47-54, 1998
Vaalamo M, Karjalainen-Lindsberg ML, Puolakkainen P, Kere J, Saarialho-Kere U: Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13, macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am J Pathol 4:1005-1014, 1998
Salmela MT, MacDonald TT, Black D, Irvine B, Zhuma T, Saarialho-Kere U, Pender SL: Upregulation of matrix metalloproteinases during T-cell mediated injury in the gut—Analysis by gene array and in situ hybridization. Gut 51:540-547, 2002
Cossins J, Dudgeon TJ, Catlin G, Gearing JA, Clements JM: Identification of MMP-18, a putative novel human matrix metalloproteinase. Biochem Biophys Res Commun 228:494-498, 1996
Pendas A, Knäuper V, Puente XS, Llano E, Mattei MG, Apte S, Murphy G, Lopez-Otin C: Identification and characterization of a novel human matrix metalloproteinase with unique structural characteristics, chromosomal location and tissue distribution. J Biol Chem 272:4281-4286, 1997
Sedlacek R, Mauch S, Kolb B, Schatzlein C, Eibel H, Peter HH, Schmitt J, Krawinkel U: Matrix metalloproteinase MMP-19 (RASI-1) is expressed on the surface of activated peripheral blood mononuclear cells and is detected as an autoantigen in rheumatoid arthritis. Immunobiology 198:137-143, 1998
Kolb C, Mauch S, Krawinkel U, Sedlacek R: Matrix metalloproteinase-19 in capillary andothelial cells: expression in acutely, but not in chronically, inflamed synovium. Exp Cell Res 250:122-130, 1999
Djonov V, Högger K, Sedlacek R, Laissue J, Draeger A: MMP-19: cellular localization of a novel metalloproteinase within normal breast tissue and mammary gland tumours. J Pathol 195:147-155, 2001
Mauch S, Kolb C, Kolb B, Sadowski T, Sedlacek R: Matrix metalloproteinase-19 is expressed in myeloid cells in an adhesion dependent manner and associates with the cell surface. J Immunol 3:1244-1251, 2002
Stracke J, Hutton M, Stewart M, Pendas AM, Smith B, Lopez-Otin C, Murphy G, Knauper V: Biochemical characterization of the catalytic domain of human matrix metalloproteinase 19. Evidence for a role as a potent basement membrane degrading enzyme. J Biol Chem 275:14809-14816, 2000
Stracke J, Fosang JA, Last K, Mercuri FA, Pendas AM, Llano E, Perris R, DiCesare Pe, Murphy G, Knauper V: Matrix metalloproteinases 19 and 20 cleave aggrecan and cartilage oligomeric matrix protein (COMP). FEBS Lett 478:52-65, 2000
de Coignac A, Elson G, Delneste Y, Magistrelli G, Jeannin P, Aubry JP, Berthier O, Schmitt D, Bonnefoy JY, Gauchat JF: Cloning of MMP-26: a novel matrilysin-like proteinase. Eur J Biochem 267:3323-3329, 2000
Park H, Ni J, Gerkema FE, Liu D, Belozerov VE, Sang QX: Identification and characterization of human endometase (MMP-26) from endometrial tumor. J Biol Chem 275:20540-20544, 2000
Marchenko G, Ratnikov BI, Rozanov DV, Godzik A, Deryugina EI, Strongin AY: Characterization of matrix metalloproteinase-26, a novel metalloproteinase widely expressed in cancer cells of epithelial origin. Biochem J 356:705-718, 2001
Zhang J, Cao Y-J, Zhao Y-G, Sang Q-X, Duan E-K: Expression of matrix metalloproteinase-26 and tissue inhibitor of metalloproteinase-4 in human normal cytotrophoblast cells and a choriocarcinoma cell line, JEG-3. Mol Hum Rep 7:659-666, 2002
Isaka K, Nishi H, Nakai H, Nakada T, Feng Li Y, Ebihara Y, Takayama: Matrix metalloproteinase-26 is expressed in human endometrium but not in endometrial carcinoma. Cancer 97:79-89, 2003
Zhao Y-G, Xiao A-Z, Newcomer RG, Park HI, Kang T, Chung LWK, Swanson MG, Zhau HE, Kurhanewicz J, Sang QA: Activation of pro-gelatinase B by endometase-/Matrilysin-2 promotes invasion of human prostate cancer cells. J Biol Chem 278:15056-15064, 2003
Illman SA, Keski-Oja J, Lohi J: Promoter characterization of the human and mouse epilysin (MMP-28) genes. Gene 275:185-194, 2001
Marchenko GN, Strongin AY: MMP-28, a new human matrix metalloproteinase with an unusual cysteine-switch sequence is widely expressed in tumors. Gene 265:87-93, 2001
Saarialho-Kere U, Kerkelä E, Jahkola T, Suomela S, Keski-Oja J, Lohi J: Epilysin (MMP-28) expression is associated with cell proliferation during epithelial repair. J Invest Dermatol 119:14-21, 2002
Saarialho-Kere UK, Vaalamo M, Puolakkainen P, Airola K, Parks WC, Karjalainen-Lindsberg ML: Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am J Pathol 2:19-526, 1996
Salmela MT, Pender SLF, Reunala T, MacDonald T, Saarialho-Kere U: Parallel expression of macrophage metalloelastase (MMP-12) in duodenal and skin lesions of patients with dermatitis herpetiformis. Gut 48:96-502, 2001
Pender SL, Salmela MT, Monteleone G, Schnapp D, McKenzie C, Spencer J, Fong S, Saarialho-Kere U, MacDonald TT: Ligation of alpha4β1 integrin on human intestinal mucosal mesenchymal cells selectively up-regulates membrane type-1 matrix metalloproteinase and confers a migratory phenotype. Am J Pathol 6:955-1962, 2000
Impola U, Toriseva M, Suomela S, Jeskanen L, Hieta N, Jahkola T, Grenman R, Kahari VM, Saarialho-Kere U: MMP-19 is expressed by proliferating epithelium but disappears with neoplastic dedifferentiation. Int J Cancer 103:709-716, 2003
Li Q, Wang H, Zhao Y, Lin H, Sang QA, Zhu C: Identification and specific expression of matrix metalloproteinase-26 in rhesus monkey endometrium during early pregnancy. Mol Hum Reprod 10:34-940, 2002
Vihinen P, Kahari VM: Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer 2:57-166, 2002
Suomela S, Kariniemi A-L, Impola U, Karvonen SL, Snellman E, Uurasmaa T, Peltonen J, Saarialho-Kere U: MMP-19 is expressed by keratinocytes in psoriasis. Acta Dermatovenereol 83:108-114, 2003
Hieta N, Impola U, Lopez-Otin C, Saarialho-Kere U, Kähäri VM: Matrix metalloproteinase-19 expression in wound healing and in dermal fibroblasts. J Invest Dermatol 121:997-1004, 2003
Yu WH, Woessner JF Jr: Heparan sulfate proteoglycans as extracellular docking molecules for matrilysin (matrix metalloproteinase 7). J Biol Chem 275:183-4191, 2000
von Bredow DC, Nagle RB, Bowden GT, Cress AE: Cleavage of beta 4 integrin by matrilysin. Exp Cell Res 236:41-345, 1997
Illman S, Keski-Oja J, Parks WC, Lohi J: Mouse Epilysin (MMP-28) is alternatively spliced and processed by a furin-like pro-protein convertase. Biochem J 375:191-197, 2003
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bister, VO., Salmela, M.T., Karjalainen-Lindsberg, ML. et al. Differential Expression of Three Matrix Metalloproteinases, MMP-19, MMP-26, and MMP-28, in Normal and Inflamed Intestine and Colon Cancer. Dig Dis Sci 49, 653–661 (2004). https://doi.org/10.1023/B:DDAS.0000026314.12474.17
Issue Date:
DOI: https://doi.org/10.1023/B:DDAS.0000026314.12474.17