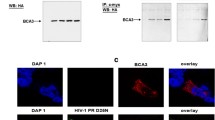
Abstract
The third reading frame of the envelope gene from HIV-1 codes for a protein homologous to the human selenoprotein glutathione peroxidase (GPX). Cells stably or transiently transfected with a HIV-1 GPX construct are protected against the loss of the mitochondrial transmembrane potential and subsequent cell death induced by exogenous reactive oxygen species (ROS) as well as mitochondrion-generated ROS. However, HIV-1 GPX does not confer a general apoptosis resistance, because HIV-1 GPX-transfected cells were not protected against cell death induced by staurosporine or oligomycin. The inhibition of cell death induced by the ROS donor tert-butylhydroperoxide was also observed in cells depleted from endogenous glutathione (GSH), suggesting that GSH is not the sole electron acceptor for HIV-1 GPX. Clinical HIV-1 isolates from long-term non-progressors (untreated patients with diagnosed HIV-1 infection for >10 years, with CD4 T cell count of >500 cells/mm3) mostly possess an intact GPX gene (with only 18% of loss-of-function mutations), while HIV-1 isolates from patients developing AIDS contain non-functional GPX mutants in 9 out of 17 cases (53%). Altogether, these data suggest that HIV-1 GPX possesses a cytoprotective, pathophysiologically relevant function.
Similar content being viewed by others
References
Fauci AS. Host factors and the pathogenesis of HIV-induced disease. Nature 1996; 384: 529-534.
Badley AD, Pilon AA, Landay A, Lynch DH. Mechanisms of HIV-associated lymphocyte apoptosis. Blood 2000; 96: 2951-2964.
Gougeon M. Cell death and immunity: Apoptosis as an HIV strategy to escape immune attack. Nat Rev Immunol 2003; 3: 392-404.
Gougeon ML, et al. Mise en évidence d'un processus d'engagement vers la mort cellulaire par apoptose dans les lymphocytes de patients infectés par le VIH. CR Acad Sci Paris Ser III Sci Vie 1991; 312: 529-535.
Groux H, et al. Activation-induced death by apoptotis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med 1992; 175: 331-340.
Gougeon ML, et al. Programmed cell death in peripheral lymphocytes from HIV-infected persons-Increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol 1996; 156: 3509-3520.
Autran B, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 1997; 277: 112-116.
Badley AD, et al. In vivo analysis of Fas/FasL interactions in HIV-infected patients. J Clin Invest 1998; 102: 79-87.
Gougeon ML, Lecouer H, Sasaki Y. Apoptosis and the CD95 system in HIV disease: Impact of highly active anti-retroviral therapy (HAART). Immunol Lett 1999; 66: 97-103.
Badley AD, et al. Dynamic correlation of apoptosis and immune activation during treatment of HIV infection. Cell Death Differ 1999; 6: 420-432.
Gougeon ML, Montagnier L. Programmed cell death as a mechanism of CD4 and CD8 T cell depletion in AIDS-Molecular control and effect of highly active anti-retroviral therapy. Ann NY Acad Sci 1999; 887: 199-212.
Macho A, et al. Mitochondrial dysfunctions in circulating T lymphocytes from human immunodeficiency virus-1 carriers. Blood 1995; 86: 2481-2487.
Moretti S, et al. Apoptosis and apoptosis-associated perturbations of peripheral blood lymphocytes during HIV infection: Comparision between AIDS patients and asymptomatic longterm non-progressors. Clin Exp Immunol 2000; 122, 364-373.
Zamzami N, et al. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med 1995; 181: 1661-1672.
Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood 2001; 98: 2603-2614.
Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med 2000; 6: 513-519.
Badley AD, Kroemer G. Mitochondrion-mediated apoptosis in HIV-1 infection. Trends Pharmacol Sci 2003; 24: 298-305.
Lum JJ, et al. Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J Clin Invest 2003; 111: 1547-1554.
Jacotot E, et al. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J Exp Med 2000; 191: 33-45.
Brenner C, Kroemer G. The mitochondriotoxic domain of Vpr determines HIV-1 virulence. J Clin Invest 2003; 111: 1455-1457.
Aukrust P, et al. Increased levels of oxidized glutathione in CD4+ lymphocytes associated with disturbed intracellular redox balance in human immunodeficiency virus type 1 infection. Blood 1995; 86: 258-267.
Petit F, et al. Productive HIV-1 infection of primary CD4+ T cells induces mitochondrial membrane permeabilization leading to caspase-independent cell death. J Biol Chem 2002; 277: 1477-1487.
Macho A, et al. Glutathione depletion is an early and calcium elevation a late event of thymocyte apoptosis. J Immunol 1997; 158: 4612-4619.
Droge W, Holm E. Role of cysteine and glutathione in HIV infection and other diseases associated with muscle wasting and immunological dysfunction. FASEB J 1997; 11: 1077-1089.
Perez OD, et al. Motexafin gadolinium (Gd-Tex) selectively induces apoptosis in HIV-1 infected CD4+ T helper cells. Proc Natl Acad Sci USA 2002; 99: 2270-2274.
Piedimonte G, et al. Oxidative protein damage and degradation in lymphocytes from patients infected with human immunodeficiency virus. J Infect Dis 1997; 176: 655-664.
Staal FJT, et al. Intracellular glutathione levels in T cell subsets decrease in HIV-infected individuals. AIDS Res Hum Retrovirus 1992; 8: 305-311.
Herzenberg LA, et al. Glutathione deficiency is associated with impaired survival in HIV disease. Proc Natl Acad Sci USA 1997; 94: 1967-1972.
Muller F, et al. Virological and immunological effects of antioxidant treatment in patients with HIV infection. Eur J Clin Invest 2000; {30}: 905-914.
Breitkreutz R, et al. Improvement of immune functions in HIV infection by sulfur supplementation: Two randomized trials. J Mol Med 2000; 78: 55-62.
Baum MK, Miguez-Burbano MJ, Campa A, Shor-Posner G. Selenium and interleukins in persons infected with human immunodeficiency virus type 1. J Infect Dis 2000; {</182|: S69-73.
Zhao L, et al. Molecular modeling and in vitro activity of an HIV-1-encoded glutathione peroxidase. Proc Natl Acad Sci USA 2000; {</97}: 6356-6361.
Castedo M, et al. Sequential involvement of Cdk1, mTOR and p53 in apoptosis induced by the human immunodeficiency virus-1 envelope. EMBO J 2002; 21: 4070-4080.
Sandstrom PA, Tebbey PW, Vancleaven S, Buttke TM. Lipid hydroperoxides induce apoptosis in T-cells displaying a HIVassociated glutathione peroxidase deficiency. J Biol Chem 1994; 2: 798-801.
Gladyshev VN, Stadtman TC, Hatfield DL, Jeang KT. Levels of major selenoproteins in T cells decrease during HIV infection and low molecular mass selenium compounds increase. Proc Natl Acad Sci USA 1999; {</96}: 835-839.
Shisler JL, Senkevich TG, Berry MJ, Moss B. Ultravioletinduced cell death blocked by a selenoprotein from a human dermatotropic poxvirus. Science 1998; 279: 102-105.
Castedo M, et al. Quantitation of mitochondrial alterations associated with apoptosis. J Immunol Methods 2002; 265: 39-47.
Yoon SO, Park SJ, Chung AS. Selenite inhibits apoptosis via activation of the PI3-K/Akt pathway. Ann NY Acad Sci 2002; 973: 221-223.
Lee YC, Tang YC, Chen YH, Wong CM, Tsou AP. Seleniteinduced survival of HuH7 hepatoma cells involves activation of focal adhesion kinase-hosphatidylinositol 3-kinase-Akt pathway and Rac1. J Biol Chem 2003; 278: 39615-9624.
Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: Central role of complex III. J Biol Chem 2003; 278: 36027-36031.
Westendorp MO, et al. HIV-1 Tat potentiates TNF-induced NF-kB activation and cytotoxicity by altering the cellular redox state. EMBO J 1995; 14: 546-554.
Jacotot E, et al. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 Vpr and Bcl-2. J Exp Med 2001; 193: 509-520.
Graham BH, et al. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat Gen 1997; 16: 226-234.
Sandstrom PA, et al. Bcl-2 expression facilitates human immunodeficiency virus type 1-mediated cytopathic effects during acute spreading infections. J Virol 1996; 70: 4617-4622.
Aillet F, et al. Human immunodeficiency virus induces a dual regulation of Bcl-2, resulting in persistent infection of CD4+ T-or monocytic cell lines. J Virol 1998; 72: 9698-9705.
Guillemard E, et al. Interleukin-7 and infection itself by human immunodeficiency virus 1 favor virus persistence in mature CD4(+)CD8(?)CD3(+) thymocytes through sustained induction of Bcl-2. Blood 2001; 98: 2166-2174.
Scheller C, et al. Caspase inhibition activates HIV in latently infected cells: Role of TNF-R1 and CD95. J Biol Chem 2002; 19: 19.
Bjornstedt M, Kumar S, Bjorkhem L, Spyrou G, Holmgren A. Selenium and the thioredoxin and glutaredoxin systems. Biomed Environ Sci 10, 271-279 (1997).
Takebe G, et al. A comparative study on the hydroperoxide and thiol specificity of the glutathione peroxidase family and selenoprotein P. J Biol Chem 2002; 277: 41254-41258.
Rover LJ, Kubota LT, Hoehr NF. Development of an amperometric biosensor based on glutathione peroxidase immobilized in a carbodiimide matrix for the analysis of reduced glutathione from serum. Clin Chim Acta 2001; 308: 55-67.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cohen, I., Boya, P., Zhao, L. et al. Anti-apoptotic activity of the glutathione peroxidase homologue encoded by HIV-1. Apoptosis 9, 181–192 (2004). https://doi.org/10.1023/B:APPT.0000018800.87358.ba
Issue Date:
DOI: https://doi.org/10.1023/B:APPT.0000018800.87358.ba