Abstract
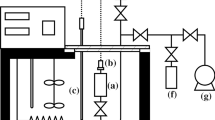
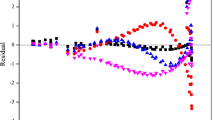
Measurements of the molar heat capacity at constant volume C v for chlorotrifluoromethane (R13) were conducted using an adiabatic method. Temperatures ranged from 95 to 338 K, and pressures were as high as 35 MPa. Measurements of vapor pressure were made using a static technique from 250 to 302 K. Measurements of (p, ρ, T) properties were conducted using an isochoric method; comprehensive measurements were conducted at 15 densities which varied from dilute vapor to highly compressed liquid, at temperatures from 92 to 350 K. The R13 samples were obtained from the same sample bottle whose mole fraction purity was measured at 0.9995. A test equation of state including ancillary equations was derived using the new vapor pressures and (p, ρ, T) data in addition to similar published data. The equation of state is a modified Benedict–Webb–Rubin type with 32 adjustable coefficients. Acceptable agreement of C v predictions with measurements was found. Published C v(ρ, T) data suitable for direct comparison with this study do not exist. The uncertainty of the C v values is estimated to be less than 2.0% for vapor and 0.5% for liquid. The uncertainty of the vapor pressures is 1 kPa, and that of the density measurements is 0.1%.
Similar content being viewed by others
REFERENCES
V. V. Altunin, V. Z. Geller, E. A. Kremenevskaya, I. I. Perelshtein, and E. K. Petrov, Thermophsical Properties of Freons Methane Series Part 2. English-language ed., T. B. Selover, Jr., ed. (Hemisphere, Washington, DC, 1987).
A. Michels, T. Wassenaar, G. J. Wolkers, C. Prins, and L. Klundert, J. Chem. Eng. Data 11:449 (1966).
H. Kubota, Y. Tanaka, and T. Makita, Rev. Phys. Chem. Japan 538 (1975).
R. C. Castro-Gomez, G. A. Iglesias-Silva, W. R. Lau, J. C. Holste, K. N. Marsh, K. R. Hall, and P. T. Eubank, personal communication (Texas A&M University, College Station, 1986).
L. F. Albright and J. J. Martin, Ind. Eng. Chem. 44:188 (1952).
K. Oguchi, I. Tanishita, K. Watanabe, T. Yamaguchi, and A. Sasayama, Bull. JSME 18:1448 (1975).
L. Reidel, Ztschr. ges. Ka-lte-Ind. 48:9 (1941).
V. Z. Geller and E. G. Porichanskii, Kholodilnaya Tekhn. Tekhnol. 29:43 (1979).
E. Fernandez-Fassnacht and F. del Rio, Cryogenics 25:204 (1985).
D. S. Rasskazov, E. K. Petrov, and E. R. Ushmaikin, Teplofiz. Svoistva Veshchestv Material. 14:7 (1980).
K. Oguchi, I. Tanishita, K. Watanabe, T. Yamaguchi, and A. Sasayama, Bull. JSME 18:1456 (1975).
H. P. Jaeger, Die Kalte 7:276 (1973).
R. D. Goodwin, J. Res. Natl. Bur. Stand. (US) C 65:231 (1961).
J. W. Magee and J. F. Ely, Int. J. Thermophys. 9:547 (1988).
J. W. Magee, J. Res. Natl. Inst. Stand. Tech. 96:725 (1991).
H. Preston-Thomas, Metrologia 27:3 (1990).
R. T Jacobsen and R. B. Stewart, J. Phys. Chem. Ref. Data 2:757 (1973).
R. G. Kunz and R. S. Kapner, J. Chem. Eng. Data 14:190 (1969).
B. Schramm and R. Gehrmann, Unpublished data communicated to J. H. Dymond and E. B. Smith, The Virial Coefficients of Pure Gases and Mixtures (Clarendon Press, Oxford, 1980).
W. Rathjen and J. Straub, Wärme-und Stoffübertragung 14:59 (1980).
V. Z. Geller and E. G. Porichanskii, Kholodilnaya Tekn. 2:42 (1980).
R. D. Goodwin and W. M. Haynes, Natl. Bur. Stand. (U.S.) Monograph 170 (U.S. GPO, Washington, DC, 1982).
S. S. Chen, R. C. Wilhoit, and B. J. Zwolinski, J. Phys. Chem. Ref. Data 5:571 (1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Magee, J.W., Outcalt, S.L. & Ely, J.F. Molar Heat Capacity Cv, Vapor Pressure, and (p, ρ, T) Measurements from 92 to 350 K at Pressures to 35 MPa and a New Equation of State for Chlorotrifluoromethane (R13). International Journal of Thermophysics 21, 1097–1121 (2000). https://doi.org/10.1023/A:1026446004383
Issue Date:
DOI: https://doi.org/10.1023/A:1026446004383