Abstract
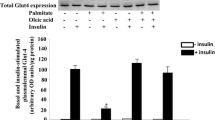
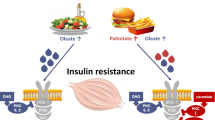
Insulin resistance is defined as the decrease in the glucose disposal in response to insulin by the target tissues. High concentrations of nonesterified fatty acids (NEFA) in plasma have been implicated with many insulin resistance states. We evaluated several aspects of the insulin resistance induced by palmitic acid in rats and found that after treatment with 0.09 g/kg of palmitic acid there is a delay in the curve of tolerance to glucose. We measured the changes in protein phosphorylation in samples from abdominus rectus muscle and there was a decrease of 64 and 75% in the levels of phosphorylation in tyrosine of the insulin receptor and insulin receptor substrate-1, respectively. This diminution in the tyrosine phosphorylation is consistent with a decrease in the main pathway known to be activated after insulin treatment, the mitogen activated protein kinases (MAPKs). If the animals were treated with inhibitors of PKC, like sphingosine, there was a prevention of the effect of palmitic acid determined at the level of tyrosine phosphorylation. According with this result, we found an increase in the phosphorylations in serine of the insulin receptor after the treatment with palmitate. These results suggest that PKC has a role as negative regulator (by phosphorylation in serine) of the insulin receptors activation in the insulin resistance induced by palmitic acid.
Similar content being viewed by others
References
Reaven GM: Role of insulin resistance in human disease. Diabetes 37: 1595-1607, 1988
Boden G: Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46: 3-10, 1997
Randle PJ, Garland PB, Hales CN, Newsholme EA: The glucose fatty acid cycle its role in insulin sensitivity and the metabolic disturbance of diabetes mellitus. Lancet 1: 785-789, 1963
Bevilacqua S, Bonadonna R, Buzzigoli G, Boni C, Ciociaro D, Maccari F, Giorico MA, Ferrannini E: Acute elevation of free fatty acid levels leads to hepatic insulin resistance in obese subjects. Metabolism 36: 502-506, 1987
Saloranta C, Koivisto V, Widen E, Falholt K, DeFronzo RA, Harkonen M, Groop L: Contribution of muscle and liver to glucose-fatty acid cycle in humans. Am J Physiol 264: E599-E605, 1993
Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GA: Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97: 2859-2865, 1996
Boden G, Chen X, Ruiz J, White JV, Rossetti L: Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest 93: 2438-2446, 1994
Whitehead JP, Clark SF, Urso B, James DE: Signaling trough the insulin receptor. Curr Opin Cell Biol 12: 222-228, 2000
Czech MP, Corvera S: Signaling mechanisms that regulate glucose transport. J Biol Chem 274: 1865-1868, 1999
Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI: Effects of free fatty acids on glucose transport and IRS-1 associated phosphatidylinositol 3-kinase activity. J Clin Invest 103: 253-259, 1999
Schmitz-Peiffer C: Signaling aspects of insulin resistance in skeletal muscle: Mechanisms induced by lipid oversupply. Cell Signal 12: 583-594, 2000
Considine RV, Caro JF: Protein kinase C: Mediator or inhibitor of insulin action? J Cell Biochem 52: 8-13, 1993
Takayama S, White MF, Kahn MF: Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J Biol Chem 263: 3440-3457, 1988
Chin JE, Dickens M, Tavare JM, Roth RA: Over expression of protein kinase C isoenzymes alpha, beta I, gamma and epsilon in cells over expressing the insulin receptor. J Biol Chem 268: 6338-6347, 1993
Muller HK, Kellerer M, Ermel B, Muhlhofer A, Obermaier-Kusser B, Vogt B, Haring HU: Prevention by protein kinase C inhibitors of glucose-induced insulin-receptor tyrosine kinase resistance in rat fat cells. Diabetes 40: 1440-1448, 1991
Considine RV, Nyce MR, Allen LE, Morales LM, Triester S, Serrano J, Colberg J, Lanzacoby S, Caro JF: Protein kinase C is increased in the liver of humans and rats with non-insulin-dependent diabetes mellitus: Alterations not due to hyperglycemia. J Clin Invest 95: 2938-2944, 1995
Avignon A, Yamada K, Zhou X, Spencer B, Cardona O, Saba-Siddique S, Galloway L, Standaert ML, Farese RV: Chronic activation of protein kinase C in soleus muscle and tissues of insulin resistance type II diabetic Goto-Kakizaki (GK) obese/aged, and obese/zucker rats. A mechanism for inhibiting glycogen synthesis. Diabetes 45: 1396-1404, 1996
Nesher M, Boneh A: Effect of fatty acids and their acyl-CoA esters on protein kinase C activity in fibroblasts: Possible implications in fatty acid oxidation defects. Biochim Biophys Acta 1221: 66-72, 1994
Storz P, Döppler H, Werning A, Pfizenmaier K, Müller G: Cross-talk mechanisms in the development of insulin resistance of skeletal muscle cells, palmitate rather than tumor necrosis factor inhibits insulin-dependent protein kinase B (PKB)/Akt stimulation and glucose uptake. Eur J Biochem 266: 17-25, 1999
Yki-Jarvinen H, Puhakainen I, Koivisto VA: Effect of free fatty acids on glucose uptake and monoxidative glycolysis across human forearm tissues in the basal state and during insulin stimulation. J Clin Endocrinol Metab 72: 1268-1277, 1992
Rothenberg PL, Lane WS, Karasik A, Backer J, White M, Kahn CR: Protein purification and partial sequence analysis of pp185, the major substrate of the insulin receptor tyrosine kinase. J Biol Chem 266: 8302-8311, 1991
Itaya K, Ui M: Colorimetric determination of free fatty acids in biological fluids. J Lipid Res 6: 16-20, 1965
Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL: Insulin receptor phosphorylation, insulin receptor-substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J Clin Invest 95: 2195-2204, 1995
Liu F, Roth RA: Identification of serines-1035/1037 in the kinase domain of the insulin receptor as protein kinase C alpha mediated phosphorylation sites. FEBS Lett 352: 389-392, 1994
Lingamanaidu V, Ravichandran DL, Esposito JC, Quon MJ: PKC-ζ phosphorylates insulin receptor substrate-1 and impairs its ability to activate phosphatidylinositol 3-kinase in response to insulin. J Biol Chem 276: 3543-3549, 2001
Svedberg J, Björntorp P, Smith U, Lönnroth P: Free-fatty acid inhibition of insulin binding, degradation and action in isolated rat hepatocytes. Diabetes 39: 570-574, 1990
Grunfeld C, Baird KL, Kahn CR: Maintenance of 3T3-L1 cells in culture media containing saturated fatty acids decreases insulin binding and insulin action. Biochem Biophys Res Commun 103: 219-226, 1981
Kriauciunas KM, Grigorescu MF, Kahn CR: Effects of heparin on insulin binding and biological activity. Diabetes 36: 163-168, 1987
Defea K, Roth RA: Modulation of insulin receptor substrate-1 tyrosine phosphorylation and function by mitogen-activated protein kinase. J Biol Chem 272: 31400-31406, 1997
Berti L, Mosthaf L, Kroder G, Kellerer M, Tippmer S, Mushack J, Seffer E, Seedorf K, Haring HU: Glucose-induced translocation of protein kinase C isoforms in rat-1 fibroblasts is paralleled by inhibition of the insulin receptor tyrosine kinase. J Biol Chem 269: 3381-3386, 1994
Strack V, Hennige AM, Krützfeldt J, Bossenmaier B, Klein HH, Keller M, Lammers R, Häring HU: Serine residues 994 and 1023/25 are important for insulin receptor kinase inhibition by protein kinase C isoforms beta2 and theta. Diabetologia 43: 443-449, 2000
Nystrom FH, Quon MJ: Insulin signaling: Metabolic pathways and mechanisms for specificity. Cell Signal 11: 563-574, 1999
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reynoso, R., Salgado, L.M. & Calderón, V. High levels of palmitic acid lead to insulin resistance due to changes in the level of phosphorylation of the insulin receptor and insulin receptor substrate-1. Mol Cell Biochem 246, 155–162 (2003). https://doi.org/10.1023/A:1023423005187
Issue Date:
DOI: https://doi.org/10.1023/A:1023423005187